Adverse effects of cephalosporins. Cephalosporins: Generations, Uses, and Adverse Effects Explained
What are the five generations of cephalosporins. How do they differ in their spectrum of coverage. What are the common indications and adverse effects of cephalosporins. How can healthcare professionals optimize cephalosporin use.
Understanding Cephalosporins: A Powerful Class of Antibiotics
Cephalosporins are a class of beta-lactam antibiotics that play a crucial role in treating a wide range of bacterial infections. These antimicrobials are classified into five distinct generations, each with its unique spectrum of coverage against gram-positive and gram-negative bacteria. Healthcare professionals rely on cephalosporins to manage various infections, from common skin conditions to more severe cases like meningitis.
The effectiveness of cephalosporins stems from their ability to inhibit bacterial cell wall synthesis, ultimately leading to the death of the microorganisms. As we delve deeper into the world of cephalosporins, we’ll explore their mechanisms of action, indications, potential adverse effects, and the importance of proper administration.

The Five Generations of Cephalosporins: A Closer Look
Cephalosporins are categorized into five generations based on their antimicrobial spectrum and the timing of their discovery. Each generation has its own set of strengths and weaknesses when it comes to targeting specific types of bacteria.
First-Generation Cephalosporins
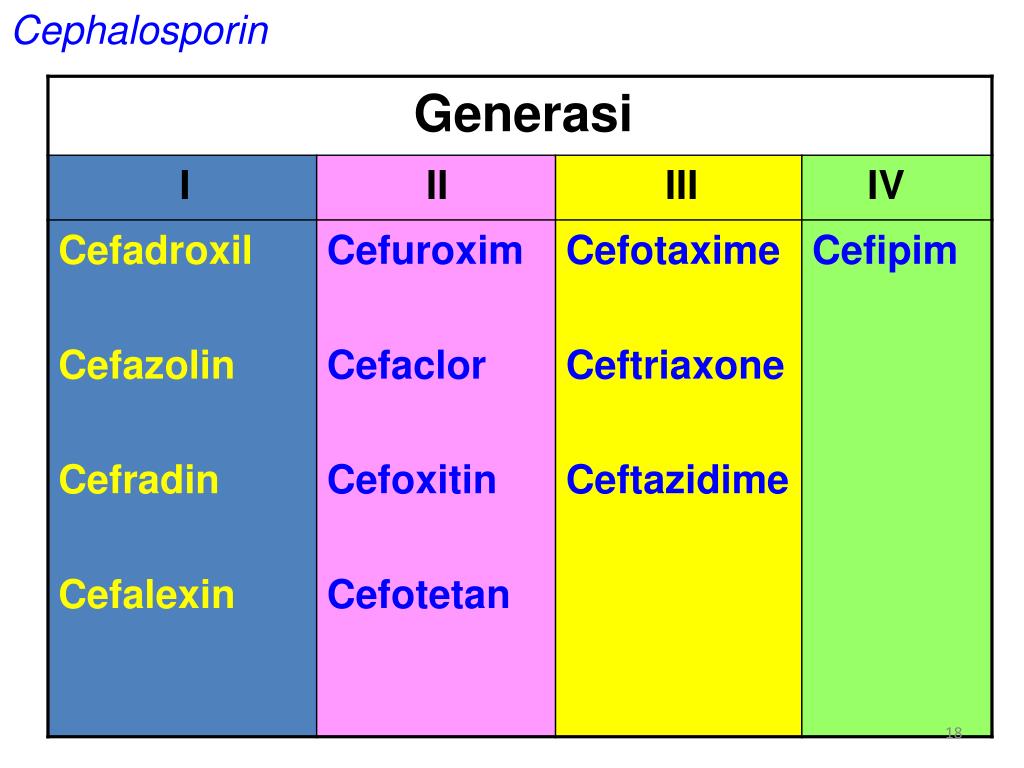
First-generation cephalosporins, including cefazolin, cephalothin, cephapirin, cephradine, cefadroxil, and cephalexin, are primarily effective against gram-positive cocci. They also have limited activity against some gram-negative bacteria such as Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae.
These antibiotics are commonly prescribed for:
- Uncomplicated skin and soft tissue infections
- Cellulitis and abscesses
- Bone infections
- Respiratory tract infections
- Genitourinary tract infections
- Biliary tract infections
- Bloodstream infections
- Otitis media
- Surgical prophylaxis
Cefazolin, in particular, is the cephalosporin of choice for surgical prophylaxis due to its efficacy and safety profile.

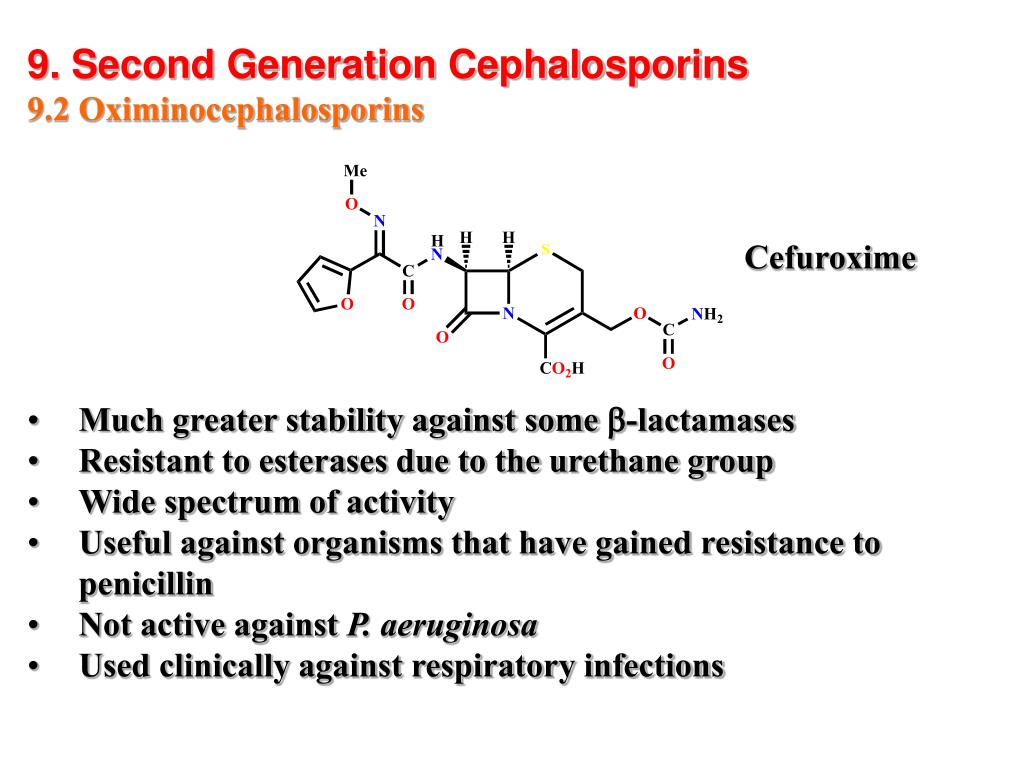
Second-Generation Cephalosporins
Second-generation cephalosporins are divided into two subgroups: the standard second-generation (e.g., cefuroxime and cefprozil) and the cephamycin subgroup (e.g., cefmetazole, cefotetan, and cefoxitin). These antibiotics have expanded coverage against gram-negative bacteria compared to their first-generation counterparts, but with slightly reduced activity against gram-positive cocci.
Key features of second-generation cephalosporins include:
- Increased coverage against Haemophilus influenzae
- Enhanced activity against Enterobacter aerogenes, Neisseria species, and Serratia marcescens
- Improved efficacy in treating respiratory infections like bronchiolitis and pneumonia
- Cefuroxime’s additional indication for Lyme disease in pregnant women and children
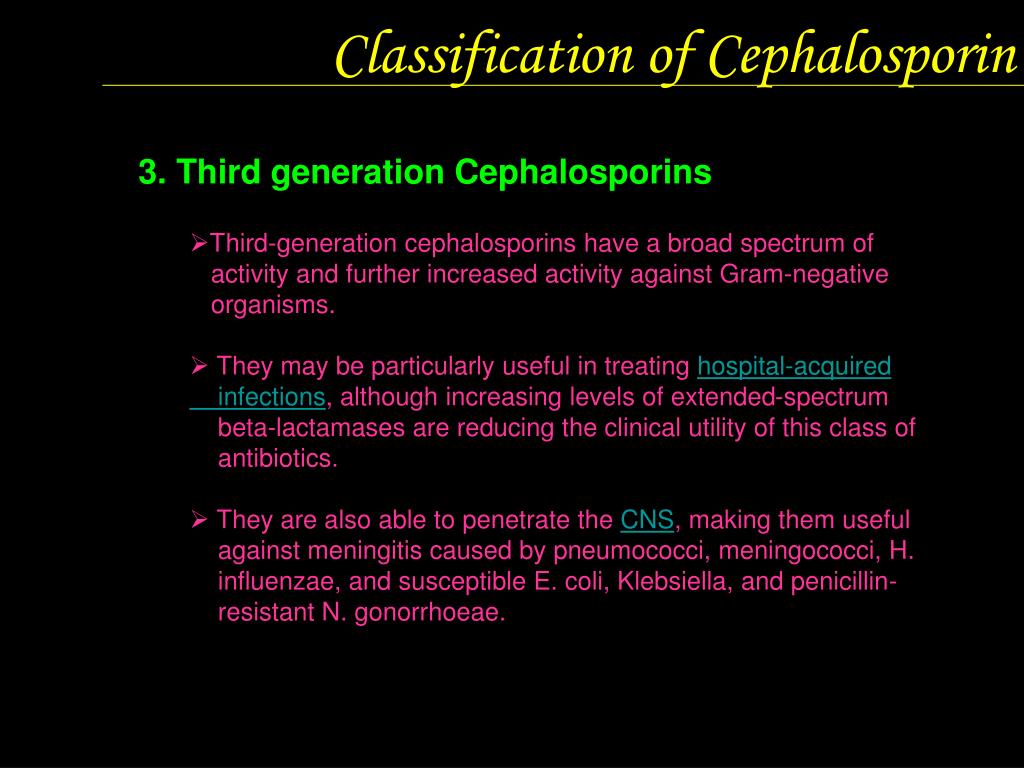
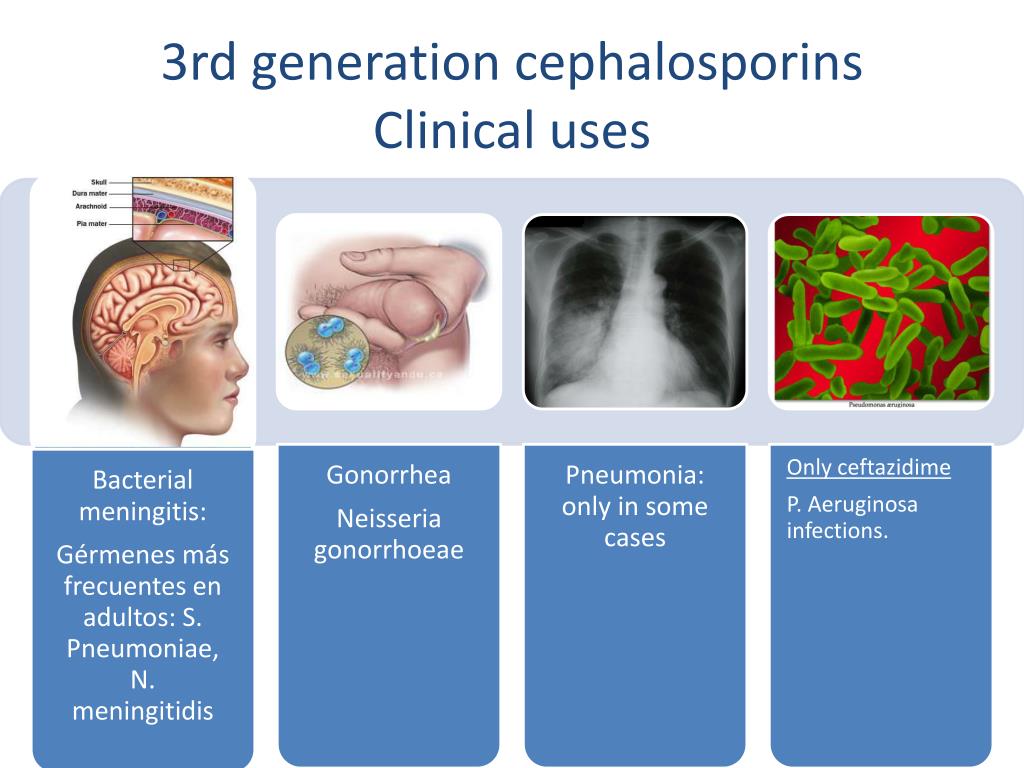
Third-Generation Cephalosporins
Third-generation cephalosporins, such as cefotaxime, ceftazidime, cefdinir, ceftriaxone, cefpodoxime, cefoperazone, and cefixime, offer extended coverage against gram-negative bacteria. They are often used to treat infections resistant to first and second-generation cephalosporins or other beta-lactam antibiotics.
Notable characteristics of third-generation cephalosporins include:
- Ability to penetrate the blood-brain barrier when administered intravenously
- Effective treatment for meningitis, particularly with ceftriaxone and cefotaxime
- Broad-spectrum activity against Enterobacteriaceae, Neisseria species, and Haemophilus influenzae
Fourth-Generation Cephalosporins
Fourth-generation cephalosporins build upon the coverage of third-generation drugs while offering additional protection against antimicrobial-resistant gram-negative bacteria. These antibiotics are particularly effective against organisms that produce beta-lactamase enzymes, which can degrade many other beta-lactam antibiotics.
Fifth-Generation Cephalosporins
The latest generation of cephalosporins is designed to combat some of the most challenging bacterial infections. Fifth-generation cephalosporins are particularly effective against:
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Penicillin-resistant Streptococcus pneumoniae
These antibiotics represent a significant advancement in the fight against antibiotic-resistant bacteria, providing healthcare professionals with additional tools to combat severe infections.
Mechanism of Action: How Cephalosporins Fight Bacteria
Cephalosporins belong to the beta-lactam family of antibiotics, which share a common structural feature called the beta-lactam ring. This ring is crucial to the antibiotic’s mechanism of action.
The process by which cephalosporins eliminate bacteria involves several steps:
- Binding to penicillin-binding proteins (PBPs) on the bacterial cell wall
- Inhibiting the final transpeptidation step of peptidoglycan synthesis
- Preventing proper cell wall formation
- Activating autolytic enzymes in the bacterial cell wall
- Causing cell lysis and death
This mechanism allows cephalosporins to be bactericidal, meaning they actively kill bacteria rather than simply inhibiting their growth.
Indications and Clinical Applications of Cephalosporins
Cephalosporins are used to treat a wide variety of bacterial infections. Their specific indications can vary depending on the generation and individual drug properties.
Common indications for cephalosporins include:
- Skin and soft tissue infections
- Respiratory tract infections (e.g., pneumonia, bronchitis)
- Urinary tract infections
- Bone and joint infections
- Meningitis (particularly with third-generation cephalosporins)
- Septicemia
- Surgical prophylaxis
- Lyme disease (in some cases)
- Gonorrhea
- Intra-abdominal infections
Can cephalosporins be used for endocarditis prophylaxis? While not FDA-approved for this purpose, first-generation cephalosporins are sometimes used off-label for endocarditis prophylaxis in susceptible patients undergoing dental or respiratory procedures.
Adverse Effects and Toxicity of Cephalosporins
While cephalosporins are generally well-tolerated, they can cause various adverse effects. Understanding these potential side effects is crucial for healthcare providers and patients alike.
Common adverse effects of cephalosporins include:
- Gastrointestinal disturbances (nausea, vomiting, diarrhea)
- Allergic reactions (rash, urticaria, anaphylaxis in severe cases)
- Hypersensitivity reactions
- Candidiasis (due to alteration of normal flora)
- Nephrotoxicity (particularly with some earlier generations)
- Hematological effects (e.g., eosinophilia, neutropenia)
- Drug-induced liver injury (rare)
Are cephalosporins safe for patients with penicillin allergies? This is a complex question. While there is some cross-reactivity between penicillins and cephalosporins, it’s generally lower than previously thought, especially with newer generations of cephalosporins. However, caution is still advised, and a thorough allergy history should be obtained before prescribing.
Contraindications and Precautions for Cephalosporin Use
While cephalosporins are widely used, there are certain situations where their use may be contraindicated or require special precautions.
Key contraindications and precautions include:
- Known hypersensitivity to cephalosporins or other beta-lactam antibiotics
- History of severe allergic reactions to penicillins (requires careful evaluation)
- Renal impairment (may require dose adjustment)
- Pregnancy and breastfeeding (most cephalosporins are considered safe, but individual assessment is necessary)
- Patients with a history of seizures (some cephalosporins may lower seizure threshold)
- Patients with gastrointestinal diseases, particularly colitis
Healthcare providers should always consider the patient’s full medical history and current condition when prescribing cephalosporins.
Optimizing Cephalosporin Use: Strategies for Healthcare Professionals
To maximize the benefits of cephalosporins while minimizing risks, healthcare professionals should follow best practices in prescribing and administering these antibiotics.
Key strategies include:
- Proper selection of the appropriate cephalosporin based on the suspected pathogen and local resistance patterns
- Accurate dosing and administration, considering patient factors such as age, weight, and renal function
- Monitoring for adverse effects and adjusting treatment as necessary
- Educating patients about potential side effects and the importance of completing the full course of antibiotics
- Implementing antimicrobial stewardship programs to promote judicious use of cephalosporins and prevent the development of resistance
- Regular review and updating of institutional guidelines for cephalosporin use
- Collaboration between different healthcare disciplines (e.g., physicians, pharmacists, microbiologists) to optimize treatment strategies
How can healthcare teams improve communication regarding cephalosporin use? Implementing regular interdisciplinary meetings, utilizing electronic health records for seamless information sharing, and establishing clear protocols for antibiotic use and monitoring can significantly enhance team communication and patient care.
Future Directions in Cephalosporin Research and Development
The field of cephalosporin research continues to evolve as scientists and pharmaceutical companies work to address the growing challenge of antibiotic resistance.
Current areas of focus in cephalosporin research include:
- Development of new cephalosporin compounds with enhanced activity against resistant bacteria
- Exploration of novel drug delivery methods to improve efficacy and reduce side effects
- Investigation of combination therapies involving cephalosporins and other antimicrobial agents
- Research into the long-term ecological impacts of cephalosporin use on microbial communities
- Studies on optimizing dosing regimens to minimize the development of resistance
What role will cephalosporins play in combating future infectious disease threats? As antibiotic resistance continues to pose a global health challenge, cephalosporins, particularly newer generations, are likely to remain important tools in the antimicrobial arsenal. However, their effectiveness will depend on judicious use and ongoing research to stay ahead of evolving bacterial resistance mechanisms.
In conclusion, cephalosporins represent a vital class of antibiotics that have revolutionized the treatment of bacterial infections. From their discovery to the development of five distinct generations, these drugs have continuously evolved to meet the changing landscape of infectious diseases. While they offer powerful antimicrobial activity, the use of cephalosporins also comes with responsibilities. Healthcare professionals must balance the benefits of these drugs with potential risks, always striving for optimal patient outcomes while preserving the effectiveness of these crucial antibiotics for future generations.
Cephalosporins – StatPearls – NCBI Bookshelf
Continuing Education Activity
Cephalosporins are beta-lactam antimicrobials used to manage a wide range of infections from gram-positive and gram-negative bacteria. The five generations of cephalosporins are useful against skin infection, resistant bacteria, meningitis, and other infections. This activity describes the indications, contraindications, and possible adverse effects of cephalosporins and will highlight the mechanism of action, adverse event profile, monitoring, route of administration, as well as other key factors.
Objectives:
Identify the mechanism of action of cephalosporins.
Describe the contraindications of cephalosporins.
Review the toxicity of cephalosporins.
Summarize interprofessional team strategies for improving care coordination and communication to advance cephalosporins and improve outcomes.
Access free multiple choice questions on this topic.
Indications
Cephalosporins are antimicrobials grouped into five generations based on their spectrum of coverage against gram-positive and gram-negative bacteria and their temporal discovery. First-generation cephalosporins have coverage against most gram-positive cocci as well as some gram-negative bacteria, e.g., Escherichia coli (E. coli), Proteus mirabilis, and Klebsiella pneumoniae. Second-generation cephalosporins have coverage against Haemophilus influenzae (H. influenzae), Moraxella catarrhalis, and Bacteroides spp. Third-generation cephalosporins have less coverage against most gram-positive organisms but have increased coverage against Enterobacteriaceae, Neisseria spp., and H. influenzae. Fourth-generation cephalosporins have similar coverage as third-generation cephalosporins but with additional coverage against gram-negative bacteria with antimicrobial resistance, e.g., beta-lactamase. Fifth-generation cephalosporins have coverage against methicillin-resistant staphylococci and penicillin-resistant pneumococci.
First-generation cephalosporins include cefazolin, cephalothin, cephapirin, cephradine, cefadroxil, and cephalexin. First-generation cephalosporins have active coverage against most gram-positive cocci, such as staphylococci
spp. and streptococci
spp., while having minimal coverage against gram-negative bacteria. Gram-negative bacteria that are more susceptible to first-generation cephalosporins are Proteus mirabilis, E. coli, and Klebsiella pneumoniae. Oral first-generation cephalosporins are commonly prescribed to use against uncomplicated skin and soft tissue infections such as cellulitis and abscesses commonly due to a Staphylococci
spp. or Streptococci
spp. infection. Additionally, clinicians can use them for bone, respiratory tract, genitourinary tract, biliary tract, bloodstream infection, otitis media, and surgical prophylaxis. In fact, cefazolin is the cephalosporin of choice for surgical prophylaxis. One of the non-FDA-approved indications is to use first-generation cephalosporins for endocarditis prophylaxis for those who are susceptible and undergoing a dental or respiratory procedure.[1][2][3]
One of the non-FDA-approved indications is to use first-generation cephalosporins for endocarditis prophylaxis for those who are susceptible and undergoing a dental or respiratory procedure.[1][2][3]
Second-generation cephalosporins divide into two subgroups: the second-generation and the cephamycin subgroup. Some of the second-generation subgroups include cefuroxime and cefprozil. The cephamycin subgroup includes cefmetazole, cefotetan, and cefoxitin. Within the first subgroup, cefuroxime has increased coverage against H. influenzae. Indications for cefuroxime also include Lyme disease in pregnant women and children. The cephamycin subgroup has increased coverage against Bacteroides species. Second-generation cephalosporins have less activity against gram-positive cocci than first-generation cephalosporins but have increased activity against gram-negative bacilli. They are often prescribed to treat respiratory infections such as bronchiolitis or pneumonia. Other indications for second-generation cephalosporins are similar to first-generation indications (bone, respiratory tract, genitourinary tract, biliary tract, bloodstream infection, otitis media, and surgical prophylaxis). In addition to the gram-negative bacteria covered by first-generation cephalosporins, second-generation cephalosporins also have coverage against H. influenzae, Enterobacter aerogenes, Neisseria species, and Serratia marcescens.[4]
In addition to the gram-negative bacteria covered by first-generation cephalosporins, second-generation cephalosporins also have coverage against H. influenzae, Enterobacter aerogenes, Neisseria species, and Serratia marcescens.[4]
Third-generation cephalosporins include cefotaxime, ceftazidime, cefdinir, ceftriaxone, cefpodoxime, cefoperazone, and cefixime. This generation has extended gram-negative bacteria coverage often used to treat gram-negative infections resistant to the first and second generation or other beta-lactam antimicrobials. When given IV, third-generation can penetrate the blood-brain barrier and cover bacteria in the cerebral spinal fluid, especially ceftriaxone and cefotaxime. Ceftriaxone can be given to treat meningitis caused by H. influenzae, Neisseria meningitidis, or Streptococcus pneumoniae. Ceftriaxone is also used to treat gonorrhea and disseminated Lyme disease. Ceftazidime, very importantly, has Pseudomonas
aeruginosa coverage. [5]
[5]
Fourth-generation cephalosporin includes cefepime. Cefepime is a broad-spectrum antimicrobial that can penetrate the cerebral spinal fluid. Cefepime has an additional quaternary ammonium group, allowing them to better penetrate the outer membrane of gram-negative bacteria. Similar to the activity of cefotaxime and ceftriaxone, cefepime can cover Streptococcus pneumoniae and methicillin-sensitive Staphylococcus aureus (MSSA). Similar to ceftazidime, cefepime, very importantly, can cover for Pseudomonas
aeruginosa. In addition to the gram-negative bacteria that third-generation covers (Neisseria spp., H. influenzae, and Enterobacteriaceae), cefepime can provide coverage against beta-lactamase-producing gram-negative bacilli. Although effective against both gram-positive and gram-negative bacteria, cefepime is reserved for serious systemic infection in patients who are likely to have multi-resistance organisms.[6]
Fifth-generation cephalosporins include ceftaroline. Ceftaroline is also a broad-spectrum antimicrobial and thus can cover susceptible gram-positive and gram-negative organisms. However, what makes it unique from the rest of the cephalosporins is that it has coverage against methicillin-resistant Staphylococcus aureus (MRSA). Ceftaroline can also cover Listeria
Ceftaroline is also a broad-spectrum antimicrobial and thus can cover susceptible gram-positive and gram-negative organisms. However, what makes it unique from the rest of the cephalosporins is that it has coverage against methicillin-resistant Staphylococcus aureus (MRSA). Ceftaroline can also cover Listeria
monocytogenes and Enterococcus faecalis. However, ceftaroline does not cover Pseudomonas aeruginosa.[7]
Mechanism of Action
Bacteria synthesize a cell wall that is strengthened by cross-linking peptidoglycan units via penicillin-binding proteins (PBP, peptidoglycan transpeptidase). Initially derived from the fungus Cephalosporium sp., cephalosporins are a large group of bactericidal antimicrobials that work via their beta-lactam rings. The beta-lactam rings bind to the penicillin-binding protein and inhibit its normal activity. Unable to synthesize a cell wall, the bacteria die.
Staphylococcus aureus, which is initially susceptible to cephalosporins, can develop resistance by changing the structure of the penicillin-binding proteins. S. aureus does this by having a gene that encodes a modified penicillin-binding protein; this prevents the cephalosporin’s beta-lactam rings from inactivating the protein. The bacterium that develops this mechanism of resistance is called methicillin-resistant Staphylococcus aureus (MRSA). As indicated above, out of the five generations of cephalosporin, only the fifth generation ceftaroline has coverage against methicillin-resistant Staphylococcus aureus. Another crucial resistance mechanism is producing the enzyme beta-lactamase, which cleaves the beta-lactam ring, preventing it from attaching to the penicillin-binding proteins, e.g., peptidoglycan transpeptidase. Beta-lactamase inhibitors can be co-formulated with cephalosporins to increase their spectrum of activity, e.g., ceftazidime/avibactam and ceftolozane/tazobactam.
S. aureus does this by having a gene that encodes a modified penicillin-binding protein; this prevents the cephalosporin’s beta-lactam rings from inactivating the protein. The bacterium that develops this mechanism of resistance is called methicillin-resistant Staphylococcus aureus (MRSA). As indicated above, out of the five generations of cephalosporin, only the fifth generation ceftaroline has coverage against methicillin-resistant Staphylococcus aureus. Another crucial resistance mechanism is producing the enzyme beta-lactamase, which cleaves the beta-lactam ring, preventing it from attaching to the penicillin-binding proteins, e.g., peptidoglycan transpeptidase. Beta-lactamase inhibitors can be co-formulated with cephalosporins to increase their spectrum of activity, e.g., ceftazidime/avibactam and ceftolozane/tazobactam.
Administration
First-generation: Cefazolin, cephalothin, and cephapirin are administered parenterally. The administration route for cefadroxil and cephalexin is oral. Cephradine administration can be parenteral or oral.
Cephradine administration can be parenteral or oral.
Second-generation: Cefuroxime can be administered parenterally or orally. Cefprozil administration is oral. Cefmetazole, cefotetan, and cefoxitin are administered parenterally.
Third-generation: Cefotaxime, ceftazidime, and ceftriaxone administration is via the parenteral route. Cefdinir, cefixime, and cefpodoxime are administered orally. A single intramuscular shot of 125 or 250 mg of ceftriaxone effectively treats uncomplicated gonococcal infection or its complications, such as pelvic inflammatory disease or epididymo-orchitis.[8][9][10]
Fourth-generation: Cefepime is administered parenterally.
Fifth-generation: Ceftaroline is administered parenterally.
Many of the parenterally administered cephalosporins have short half-lives and need to be given more frequently in patients with normal renal function. Cefazolin and ceftriaxone have a longer half-life; thus, they do not need to be dosed as often. Ceftriaxone is the only cephalosporin that does not need to have its dose modified in the presence of renal failure. However, in patients with both renal and hepatic impairment, the recommended daily dose should not exceed 2 g.[11]
However, in patients with both renal and hepatic impairment, the recommended daily dose should not exceed 2 g.[11]
Adverse Effects
Cephalosporins have low toxicity and are generally safe. The most common adverse reactions from cephalosporins are nausea, vomiting, lack of appetite, and abdominal pain.
The less common adverse reaction includes:
Hypersensitivity Reaction
A hypersensitivity reaction to cephalosporin is infrequent and is more common in first and second-generation cephalosporins. Common allergic reaction to cephalosporin includes rash, hives, and swelling. Rarely will the hypersensitivity reaction result in anaphylaxis. Patients who are allergic to penicillin might show a hypersensitive reaction to cephalosporins as well. This cross-reactivity is more common in first and second-generation cephalosporins because they have R-groups more similar to penicillin G. Third generation and beyond show minimal cross-reactivity.[12][13]
Drug-induce Immune Hemolytic Anemia (DIIHA)
The proposed mechanism of action of DIIHA is that the drug binds to the red blood cell membrane; this causes no harm to the red blood cell itself or the patient. However, if the patient starts making IgG antibodies against the drug, the antibody will bind to the red blood cell. The immune system will react with the abnormal red blood cell resulting in hemolysis. Cefotetan and ceftriaxone are the two cephalosporins most likely to cause DIIHA.[14]
However, if the patient starts making IgG antibodies against the drug, the antibody will bind to the red blood cell. The immune system will react with the abnormal red blood cell resulting in hemolysis. Cefotetan and ceftriaxone are the two cephalosporins most likely to cause DIIHA.[14]
Disulfiram-like Reaction
Cephalosporins containing a methyltetrazolethiol side chain can inhibit the aldehyde dehydrogenase enzyme resulting in the accumulation of acetaldehyde. Cefamandole, cefoperazone, and moxalactam are the most common cephalosporin to present with this reaction.[15]
Vitamin K Deficiency
Certain cephalosporins can inhibit vitamin K epoxide reductase, preventing the production of the reduced(active) vitamin K. Therefore, there is a decreased synthesis of coagulation factors, and the patient is predisposed to hypoprothrombinemia.[16]
Increase Nephrotoxicity of Aminoglycosides
There are reported cases of drug-induced nephrotoxicity when patients take cephalosporin and aminoglycosides in combination, but other factors often cloud the evidence.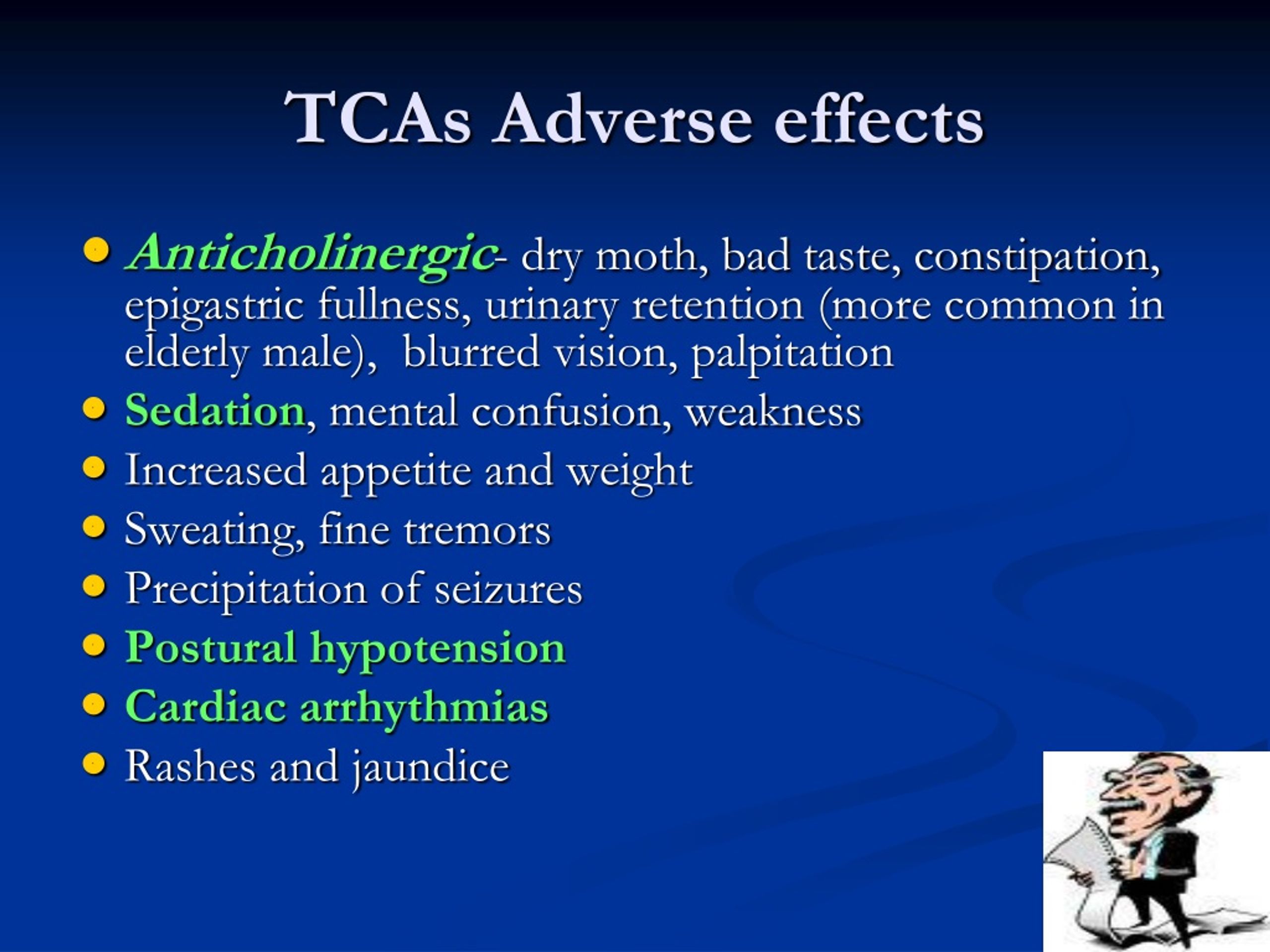 Therefore, the synergistic nephrotoxicity of cephalosporin and aminoglycoside is not to be completely understood.[17][18]
Therefore, the synergistic nephrotoxicity of cephalosporin and aminoglycoside is not to be completely understood.[17][18]
Pseudomembranous Colitis
Pseudomembranous colitis is often associated with the use of clindamycin and ampicillin. Cephalosporin use is also a common cause of pseudomembranous colitis, especially third-generation cephalosporins.[19][17]
Contraindications
One of the contraindications of cephalosporin is if patients are allergic to them or have had an anaphylactic reaction to penicillin or other beta-lactam antimicrobials.
Ceftriaxone is contraindicated in neonates with hyperbilirubinemia because of reports that ceftriaxone displaces bilirubin from albumin, increasing the free bilirubin concentrations and increasing the risk of jaundice in neonates.[20][21] Ceftriaxone reacts to a calcium-containing solution, and it can precipitate in the lungs and kidneys of infants less than 28 days old, which could be life-threatening. Therefore, ceftriaxone is also contraindicated in infants less than 28 days old if they are expected to receive any calcium-containing products. [22]
[22]
Monitoring
It is essential to monitor for possible signs of anaphylactic reaction as well as allergic reactions such as hives, itching, and swelling. Physicians and pharmacists also need to monitor renal function periodically because that could potentially warrant a change in the dose and/or dosing frequency of the cephalosporin (except for ceftriaxone).[23] With other possible adverse reactions listed above, monitor CBC for possible signs of drug-induced immune hemolytic anemia or hypoprothrombinemia from vitamin K deficiency. Also, monitor for possible signs of a disulfiram-like reaction or pseudomembranous colitis.
Toxicity
Testing the effects of high dosage cephalosporin in rabbits, there is new evidence of nephrotoxicity due to its effect on the mitochondria system of the kidney.[24] Cefepime overdose can result in seizures and encephalopathy. Studies show it to potentially result from cefepime crossing the blood-brain barrier and displaying concentration-dependent ϒ-aminobutyric acid (GABA) antagonism, which can also occur with toxic doses of penicillin G. Other studies show altered mental status and a triphasic wave discharge on electroencephalogram (EEG). Discontinuation of cefepime demonstrates normalization of mental status.[25][26]
Other studies show altered mental status and a triphasic wave discharge on electroencephalogram (EEG). Discontinuation of cefepime demonstrates normalization of mental status.[25][26]
Exercise caution with cephalosporin treatment in patients with a history of seizures, especially with poor renal function.
Enhancing Healthcare Team Outcomes
Effective interprofessional teamwork and coordination by clinicians, nurses, pharmacists, and other healthcare professionals are required to provide the best care for the patient. One of the principles that enhance healthcare team outcomes is having a shared goal for everyone, including the patient. Having clear roles between the different interprofessional team members and trusting each other can increase the team’s efficiency. Crucial for team success is having effective communication skills. A clinician needs to be able to accurately diagnose a disease and prescribe the proper medication and inform possible adverse effects to the patients. Nurses also need to know possible adverse effects so that they can inform the physician if they notice any adverse effects developing. A pharmacist can educate the patient on how to properly administer the drug and the other potential adverse effects, as well as verify agent selection and coverage and report any potential interactions to the ordering clinician. The patient also must tell the clinician and nurse what they are experiencing, anything unusual, so that everyone is informed about the patient’s well-being. Through effective interprofessional healthcare teamwork, appropriate management of cephalosporin adverse drug reactions can occur, resulting in better patient outcomes. [Level 5]
Nurses also need to know possible adverse effects so that they can inform the physician if they notice any adverse effects developing. A pharmacist can educate the patient on how to properly administer the drug and the other potential adverse effects, as well as verify agent selection and coverage and report any potential interactions to the ordering clinician. The patient also must tell the clinician and nurse what they are experiencing, anything unusual, so that everyone is informed about the patient’s well-being. Through effective interprofessional healthcare teamwork, appropriate management of cephalosporin adverse drug reactions can occur, resulting in better patient outcomes. [Level 5]
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Hsieh WC, Ho SW. Evaluation of antibacterial activities of cephalosporin antibiotics: cefazolin, cephaloridine, cephalothin, and cephalexin.
 Zhonghua Min Guo Wei Sheng Wu Xue Za Zhi. 1975 Mar;8(1):1-11. [PubMed: 1097210]
Zhonghua Min Guo Wei Sheng Wu Xue Za Zhi. 1975 Mar;8(1):1-11. [PubMed: 1097210]- 2.
Griffith RS. The pharmacology of cephalexin. Postgrad Med J. 1983;59 Suppl 5:16-27. [PubMed: 6364086]
- 3.
Bergeron MG, Brusch JL, Barza M, Weinstein L. Bactericidal activity and pharmacology of cefazolin. Antimicrob Agents Chemother. 1973 Oct;4(4):396-401. [PMC free article: PMC444566] [PubMed: 4598612]
- 4.
Tartaglione TA, Polk RE. Review of the new second-generation cephalosporins: cefonicid, ceforanide, and cefuroxime. Drug Intell Clin Pharm. 1985 Mar;19(3):188-98. [PubMed: 3884304]
- 5.
Klein NC, Cunha BA. Third-generation cephalosporins. Med Clin North Am. 1995 Jul;79(4):705-19. [PubMed: 7791418]
- 6.
Okamoto MP, Nakahiro RK, Chin A, Bedikian A, Gill MA. Cefepime: a new fourth-generation cephalosporin. Am J Hosp Pharm. 1994 Feb 15;51(4):463-77; quiz 541-2. [PubMed: 8017411]
- 7.
Zhanel GG, Sniezek G, Schweizer F, Zelenitsky S, Lagacé-Wiens PR, Rubinstein E, Gin AS, Hoban DJ, Karlowsky JA.
 Ceftaroline: a novel broad-spectrum cephalosporin with activity against meticillin-resistant Staphylococcus aureus. Drugs. 2009;69(7):809-31. [PubMed: 19441869]
Ceftaroline: a novel broad-spectrum cephalosporin with activity against meticillin-resistant Staphylococcus aureus. Drugs. 2009;69(7):809-31. [PubMed: 19441869]- 8.
Judson FN. Treatment of uncomplicated gonorrhea with ceftriaxone: a review. Sex Transm Dis. 1986 Jul-Sep;13(3 Suppl):199-202. [PubMed: 3094173]
- 9.
Jennings LK, Krywko DM. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Mar 13, 2023. Pelvic Inflammatory Disease. [PubMed: 29763134]
- 10.
Rupp TJ, Leslie SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Nov 28, 2022. Epididymitis. [PubMed: 28613565]
- 11.
Andriole VT. Pharmacokinetics of cephalosporins in patients with normal or reduced renal function. J Infect Dis. 1978 May;137 Suppl:S88-S99. [PubMed: 349098]
- 12.
Moreno E, Macías E, Dávila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf.
 2008 May;7(3):295-304. [PubMed: 18462187]
2008 May;7(3):295-304. [PubMed: 18462187]- 13.
Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013 Aug;45(1):131-42. [PubMed: 23546989]
- 14.
Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2009:73-9. [PubMed: 20008184]
- 15.
Uri JV, Parks DB. Disulfiram-like reaction to certain cephalosporins. Ther Drug Monit. 1983 Jun;5(2):219-24. [PubMed: 6224316]
- 16.
Shearer MJ, Bechtold H, Andrassy K, Koderisch J, McCarthy PT, Trenk D, Jähnchen E, Ritz E. Mechanism of cephalosporin-induced hypoprothrombinemia: relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J Clin Pharmacol. 1988 Jan;28(1):88-95. [PubMed: 3350995]
- 17.
Rankin GO, Sutherland CH. Nephrotoxicity of aminoglycosides and cephalosporins in combination. Adverse Drug React Acute Poisoning Rev.
 1989 Summer;8(2):73-88. [PubMed: 2672726]
1989 Summer;8(2):73-88. [PubMed: 2672726]- 18.
Silverblatt F. Pathogenesis of nephrotoxicity of cephalosporins and aminoglycosides: a review of current concepts. Rev Infect Dis. 1982 Sep-Oct;4 Suppl:S360-5. [PubMed: 7178755]
- 19.
de Lalla F, Privitera G, Ortisi G, Rizzardini G, Santoro D, Pagano A, Rinaldi E, Scarpellini P. Third generation cephalosporins as a risk factor for Clostridium difficile-associated disease: a four-year survey in a general hospital. J Antimicrob Chemother. 1989 Apr;23(4):623-31. [PubMed: 2663814]
- 20.
Gulian JM, Gonard V, Dalmasso C, Palix C. Bilirubin displacement by ceftriaxone in neonates: evaluation by determination of ‘free’ bilirubin and erythrocyte-bound bilirubin. J Antimicrob Chemother. 1987 Jun;19(6):823-9. [PubMed: 3610909]
- 21.
Bickford CL, Spencer AP. Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature.
 Pharmacotherapy. 2005 Oct;25(10):1389-95. [PubMed: 16185184]
Pharmacotherapy. 2005 Oct;25(10):1389-95. [PubMed: 16185184]- 22.
Bradley JS, Wassel RT, Lee L, Nambiar S. Intravenous ceftriaxone and calcium in the neonate: assessing the risk for cardiopulmonary adverse events. Pediatrics. 2009 Apr;123(4):e609-13. [PubMed: 19289450]
- 23.
Spyker DA, Thomas BL, Sande MA, Bolton WK. Pharmacokinetics of cefaclor and cephalexin: dosage nomograms for impaired renal function. Antimicrob Agents Chemother. 1978 Aug;14(2):172-7. [PMC free article: PMC352429] [PubMed: 697345]
- 24.
Tune BM, Fravert D. Cephalosporin nephrotoxicity. Transport, cytotoxicity and mitochondrial toxicity of cephaloglycin. J Pharmacol Exp Ther. 1980 Oct;215(1):186-90. [PubMed: 7452482]
- 25.
Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, Fraser GL. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017 Nov 14;21(1):276. [PMC free article: PMC5686900] [PubMed: 29137682]
- 26.

Tchapyjnikov D, Luedke MW. Cefepime-Induced Encephalopathy and Nonconvulsive Status Epilepticus: Dispelling an Artificial Dichotomy. Neurohospitalist. 2019 Apr;9(2):100-104. [PMC free article: PMC6429673] [PubMed: 30915188]
Disclosure: Toai Bui declares no relevant financial relationships with ineligible companies.
Disclosure: Charles Preuss declares no relevant financial relationships with ineligible companies.
Uses, List of Generations, Side Effects, and More
Cephalosporins are a type of antibiotic that can treat a range of simple infections. People can take them orally or doctors can inject them into a vein.
Cephalosporins are a type of antibiotic. Antibiotics are medications that treat bacterial infections. There are many types, often called classes, of antibiotics available. Cephalosporins are a type of beta-lactam antibiotic.
They can be taken orally or injected into a vein (intravenous injection), depending on the infection.
Read on to learn more about cephalosporins, including what they treat and the side effects they can cause.
Healthcare providers use cephalosporins to treat a variety of bacterial infections, especially for people who are allergic to penicillin, another common antibiotic.
Some examples of infections that cephalosporins can treat include:
- skin or soft tissue infections
- urinary tract infections (UTIs)
- strep throat
- ear infections
- pneumonia
- sinus infections
- meningitis
- gonorrhea
Oral cephalosporins are generally used for simple infections that are easy to treat. For example, a routine case of strep throat might be treated with a course of oral cephalosporins.
Intravenous (IV) cephalosporins are used for more severe infections. This is because IV antibiotics reach your tissues faster, which can make a big difference if you have a serious infection, such as meningitis.
Cephalosporins are grouped together based on the type of bacteria that they’re most effective against. These groups are referred to as generations. There are five generations of cephalosporins.
These groups are referred to as generations. There are five generations of cephalosporins.
To understand the differences between the generations, it’s important to understand the difference between Gram-positive and Gram-negative bacteria.
One of the main distinctions between the two is their cell wall structure:
- Gram-positive bacteria have thicker membranes that are easier to penetrate. Think of their cell wall as a chunky, loose-knit sweater.
- Gram-negative bacteria have thinner membranes that are harder to penetrate, making them more resistant to some antibiotics. Think of their wall as a piece of fine chain mail.
First-generation cephalosporins
First-generation cephalosporins are very effective against Gram-positive bacteria. But they’re only somewhat effective against Gram-negative bacteria.
First-generation cephalosporins might be used to treat:
- skin and soft tissue infections
- UTIS
- strep throat
- ear infections
- pneumonia
Some first-generation cephalosporins are used as prophylactic antibiotics for surgery involving the chest, abdomen, or pelvis.
Examples of first-generation cephalosporins include:
- cephalexin (Keflex)
- cefadroxil (Duricef)
- cephradine (Velosef)
summary
First-generation cephalosporins are more effective against Gram-positive bacteria, though they also work against some Gram-negative bacteria.
Second-generation cephalosporins
Second-generation cephalosporins also target some types of Gram-positive and Gram-negative bacteria. But they’re less effective against certain Gram-positive bacteria than first-generation cephalosporins are.
They’re often used to treat respiratory infections, such as bronchitis or pneumonia.
Other infections sometimes treated with second-generation cephalosporins include:
- ear infections
- sinus infections
- UTIs
- gonorrhea
- meningitis
- sepsis
Examples of second-generation cephalosporins include:
- cefaclor (Ceclor)
- cefuroxime (Ceftin)
- cefprozil (Cefzil)
summary
Second-generation cephalosporins target both Gram-positive and Gram-negative bacteria.
But they’re a little less effective against Gram-positive bacteria compared to first-generation cephalosporins
Third-generation cephalosporins
Third-generation cephalosporins are more effective against Gram-negative bacteria compared to both the first and second generations. They’re also more active against bacteria that may be resistant to previous generations of cephalosporins.
The third generation also tend to be less active than previous generations against Gram-positive bacteria, including Streptococcus and Staphylococcus species.
One third-generation cephalosporin, ceftazidime (Fortaz), is often used to treat pseudomonas infections, including hot tub folliculitis.
Third-generation cephalosporins may also be used to treat:
- skin and soft tissue infections
- pneumonia
- UTIs
- gonorrhea
- menigitis
- Lyme disease
- sepsis
A few examples of third-generation cephalosporins include:
- cefixime (Suprax)
- ceftibuten (Cedax)
- cefpodoxime (Vantin)
Summary
Third-generation cephalosporins are effective against many Gram-negative bacteria and bacteria that haven’t responded to first- or second-generation cephalosporins.
Fourth-generation cephalosporins
Cefepime (Maxipime) is the only fourth-generation cephalosporin that’s available in the United States. While effective against a variety of Gram-positive and Gram-negative bacteria, it’s usually reserved for more severe infections.
Cefepime can be used to treat the following types of infections:
- skin and soft tissue infections
- pneumonia
- UTIs
- abdominal infections
- meningitis
- sepsis
Cefepime can be administered intravenously or with an intramuscular injection. It may also be given to people with a low white blood cell count, which can increase the risk of developing a severe infection.
Summary
Fourth-generation cephalosporins work against both Gram-positive and Gram-negative bacteria. They’re generally used for more severe infections or for those with weakened immune systems.
Fifth-generation cephalosporins
You may hear fifth-generation cephalosporins referred to as advanced- generation cephalosporins. There’s one fifth-generation cephalosporin, ceftaroline (Teflaro), available in the United States.
There’s one fifth-generation cephalosporin, ceftaroline (Teflaro), available in the United States.
This cephalosporin can be used to treat bacteria, including resistant Staphylococcus aureus (MRSA) and Streptococcus species, that are resistant to penicillin antibiotics.
Otherwise, ceftaroline’s activity is similar to that of third-generation cephalosporins, although it isn’t effective against Pseudomonas aeruginosa.
Summary
Ceftaroline is the only fifth-generation cephalosporin available in the United States. It’s often used to treat infections, including MRSA infections, that are resistant to other antibiotics.
As with any kind of medication, you can be allergic to cephalosporins. The most common sign of an allergic reaction to cephalosproins is a skin rash.
In rare cases, cephalosprins may cause a serious allergic reaction known as anaphylaxis.
Symptoms of anaphylaxis include:
- hives
- flushed skin
- swollen tongue and throat
- breathing difficulties
- low blood pressure
- rapid or weak pulse
- nausea or vomiting
- diarrhea
- dizziness
- fainting
get help
Anaphylaxis can be life-threatening.
Seek immediate medical treatment if you’re taking a cephalosporin and experience symptoms of anaphylaxis.
What if I’m allergic to penicillin?
It’s rare to be allergic to both penicillin and cephalosporins. But if you’ve had a serious anaphylactic reaction to penicillin antibiotics in the past, you shouldn’t take cephalosporins.
It’s uncommon to have an allergy to both penicillin antibiotics and cephalosporins, so cephalosporins can be used cautiously in people with a penicillin allergy.
However, people who’ve had a serious anaphylactic reaction to penicillin antibiotics shouldn’t take cephalosporins.
In addition, some cephalosporins are more likely to cause a reaction in people with a penicillin allergy. These include:
- cephalothin
- cephalexin
- cefadroxil
- cefazolin
Cephalosporins can cause a range of side effects, including:
- stomach upset
- nausea
- vomiting
- diarrhea
- yeast infection or oral thrush
- dizziness
One of the more serious side effects that can occur is a C. difficile infection. This infection typically occurs after a long course of antibiotics and can be potentially life-threatening.
difficile infection. This infection typically occurs after a long course of antibiotics and can be potentially life-threatening.
Symptoms to watch out for include:
- watery diarrhea
- abdominal pain
- fever
- nausea
- decreased appetite
You can help to prevent stomach upset and diarrhea by:
- taking probiotics, which can help to add good bacteria to your digestive tract
- following the instructions that come with your medication, as some antibiotics should be taken with food, while others should be taken on an empty stomach
- avoiding foods that can contribute to stomach upset, such as spicy or greasy foods
Cephalosporins are generally safe for most people, including those who are pregnant. In fact, some first-generation cephalosporins are commonly used to treat UTIs in pregnant people.
However, you shouldn’t take cephalosporins if you’re breastfeeding.
Cephalosporins can sometimes interact with other medications you’re taking. Make sure to tell your healthcare provider about all other medications you take, including supplements, vitamins, and over-the-counter medications.
Make sure to tell your healthcare provider about all other medications you take, including supplements, vitamins, and over-the-counter medications.
Cephalosporins are a type of antibiotic used to treat a range of bacterial infections. There are different generations of cephalosporins, and some are better suited to treat certain infections than others.
If you have to take antibiotics, make sure to tell your doctor about all other medications you take, as well as any previous allergic reactions to antibiotics.
Remember
Make sure you take the full course of antibiotics as prescribed by your doctor, even if you start to feel better before finishing them. Otherwise, you may not kill all of the bacteria, which can make them resistant to antibiotics.
Side effects of cephalosporins
Allergic reactions (in 1-4% of patients):
urticaria, transient eosinophilia,
rarely bronchospasm, anphylactic
shock. Precursive allergy to penicillins
rare (2% of cases).
When using high doses –
reversible hematopoiesis suppression
(leukopenia, neutropenia), bleeding.
Hypoprothrombinemia and hemorrhagic
syndrome is most characteristic of
cefamandole, cefotetan, cefoperazone,
cefmetazole, moxalactam. These same
drugs cause intolerance
alcohol.
Transient increase in activity
aminotransferases and alkaline phosphatase.
When using high doses of cephalosporins
possibly increased nephrotoxicity
combination of cephalosporins with
loop diuretics and aminoclicosides.
Dyspeptic disorders in
the use of cephalosporins that produce
bile (cefoperazone, ceftriaxone).
Monobactams
Aztreonam.
The basis of molecular structure
aztreonam, as well as other beta-lactam
antibiotics is represented by beta –
lactam ring. Mechanism: inhibition
transpeptidase enzyme followed by
disruption of the cell wall
microorganisms and their death.
Manifests
high activity towards
Gram-negative microorganisms
(Escherichia, Klebsiella, Proteus, Morganella,
sinengnoy stick, serations, neisseria,
Haemophilus influenzae, Citrobacter), and
resistance to beta-lactamases.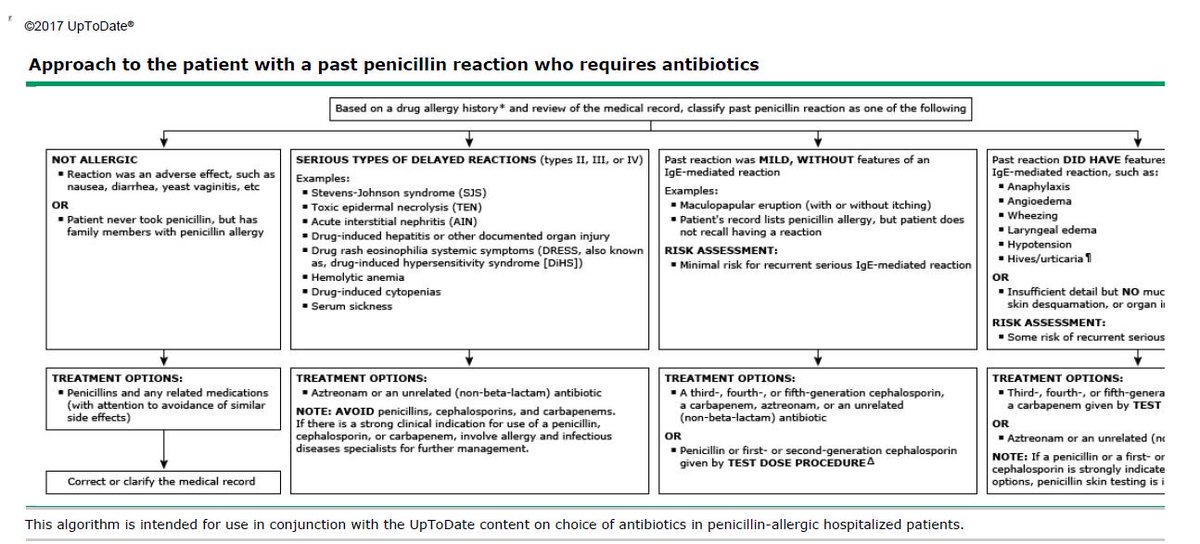 TO
TO
resistant staphylococci,
streptococci, pneumococci, bacteroids.
Unlike cephalosporins and carbapenems
does not stimulate the production of beta-lactamase
gram-negative bacteria.
Can
apply for intolerance
penicillins, cephalosporins or
restrictions on the use of aminoclicosides
(impaired kidney function, elderly
age
Carbapenems.
First time
have been isolated from Streptomuces
cattleya. Distinguish
1st generation: imepenem, tienam, primaxin:
2nd generation: meropenem.
This
highly active antibiotics. Their STK
approaching the IPC. They take first
place in terms of activity in relation to
Gram-positive microorganisms, and
for Gram-negative
microorganisms are second only to
fluoroquinolones. Carbapenems have
the broadest spectrum of action among
all currently in use
antibacterial agents: gram-positive
cocci (strepto-, pneumococci),
Gram-negative bacteria (intestinal
coli, Pseudomonas aeruginosa, meningococci,
gonococci, legionella): anaerobic
flora, including B.
Fragilis:
actinomycetes. Moderately active in
against enterococci, Pseudomonas aeruginosa
sticks, listeria. Not valid on
chlamydia, mycoplasma, tuberculosis.
Readings
for use
are severe infections
association of pathogens: infections
urinary tract, pelvis and
abdominal cavity, pneumonia, septicemia,
infections in immunocompromised patients
and agranulocytosis, etc.
From
side effects are possible dyspeptic
disorders, thrombophlebitis, eosinophilia,
pseudomembranous colitis, arterial
hypotension, increased activity
hepatic transaminases.
Efficacy and safety study of a new cephalosporin antibiotic in the treatment of acute bacterial rhinosinusitis
Efficacy and safety study of a new cephalosporin antibiotic in the treatment of acute bacterial rhinosinusitis
Website of the publishing house “Media Sfera”
contains materials intended exclusively for healthcare professionals. By closing this message, you confirm that you are a registered medical professional or student of a medical educational institution.
Savlevich E.L.
Department of Otorhinolaryngology FGBU DPO “Educational and Scientific Medical Center” of the Administration of the President of the Russian Federation, Moscow, Russia
Department of Otorhinolaryngology FSBI UNMC UD of the President of the Russian Federation
Polyclinic No. 5 of the Office of the President of the Russian Federation Moscow, Russia, 119121
Efficacy and safety of a new cephalosporin antibiotic in the treatment of acute bacterial rhinosinusitis
Authors:
Savlevich E.L., Kozlov V.S., Zharkikh M.A.
More about the authors
Journal:
Bulletin of otorhinolaryngology.
2016;81(6): 73-77
DOI:
10.17116/otorino201681673-77
How to quote:
Savlevich E.L., Kozlov V.S., Zharkikh M.A. Study of the efficacy and safety of a new cephalosporin antibiotic in the treatment of acute bacterial rhinosinusitis. Bulletin of otorhinolaryngology.
2016;81(6):73-77.
Savlevich EL, Kozlov VS, Zharkykh MA. A study of the efficacy and safety of new cephalosporin in the treatment of acute bacterial rhinosinusitis. Vestnik Oto-Rino-Laringologii. 2016;81(6):73‑77. (In Russ.)
https://doi.org/10.17116/otorino201681673-77
Read metadata
Acute rhinosinusitis occupies a leading position in the frequency of prescription of antibiotics worldwide. At the same time, the relevance of the problem of antibiotic resistance and the safety of the use of antibacterial drugs is acquiring an international dimension. The article presents the main mechanisms of bacterial resistance and the development of side effects when using macrolides, respiratory fluoroquinolones and various classes of β-lactam antibiotics. The formation of IgE-mediated allergy to cephalosporins is associated with the R1 side chain. The structure of R1 is similar to penicillins only in I and II generation cephalosporins, thereby creating conditions for the development of cross-allergy. In III and IV generation cephalosporins, R1 is represented by a qualitatively different chemical compound (aminothiazole-oxime group), which increases the level of tolerance to these classes of antibiotics. The data of an observational study on the evaluation of the efficacy and safety of a new drug Spectracef, the active substance of which is the III generation cephalosporin cefditoren, in the treatment of acute bacterial rhinosinusitis in outpatient practice are presented.
In III and IV generation cephalosporins, R1 is represented by a qualitatively different chemical compound (aminothiazole-oxime group), which increases the level of tolerance to these classes of antibiotics. The data of an observational study on the evaluation of the efficacy and safety of a new drug Spectracef, the active substance of which is the III generation cephalosporin cefditoren, in the treatment of acute bacterial rhinosinusitis in outpatient practice are presented.
Keywords:
acute rhinosinusitis
antibiotics
cephalosporins
penicillins
allergy
resistance
side effects
Authors:
Savlevich E.L.
Department of Otorhinolaryngology FGBU DPO “Educational and Scientific Medical Center” Office of the President of the Russian Federation, Moscow, Russia, 121359
Kozlov V.S.
Department of Otorhinolaryngology FSBI UNMC UD of the President of the Russian Federation
Polyclinic No.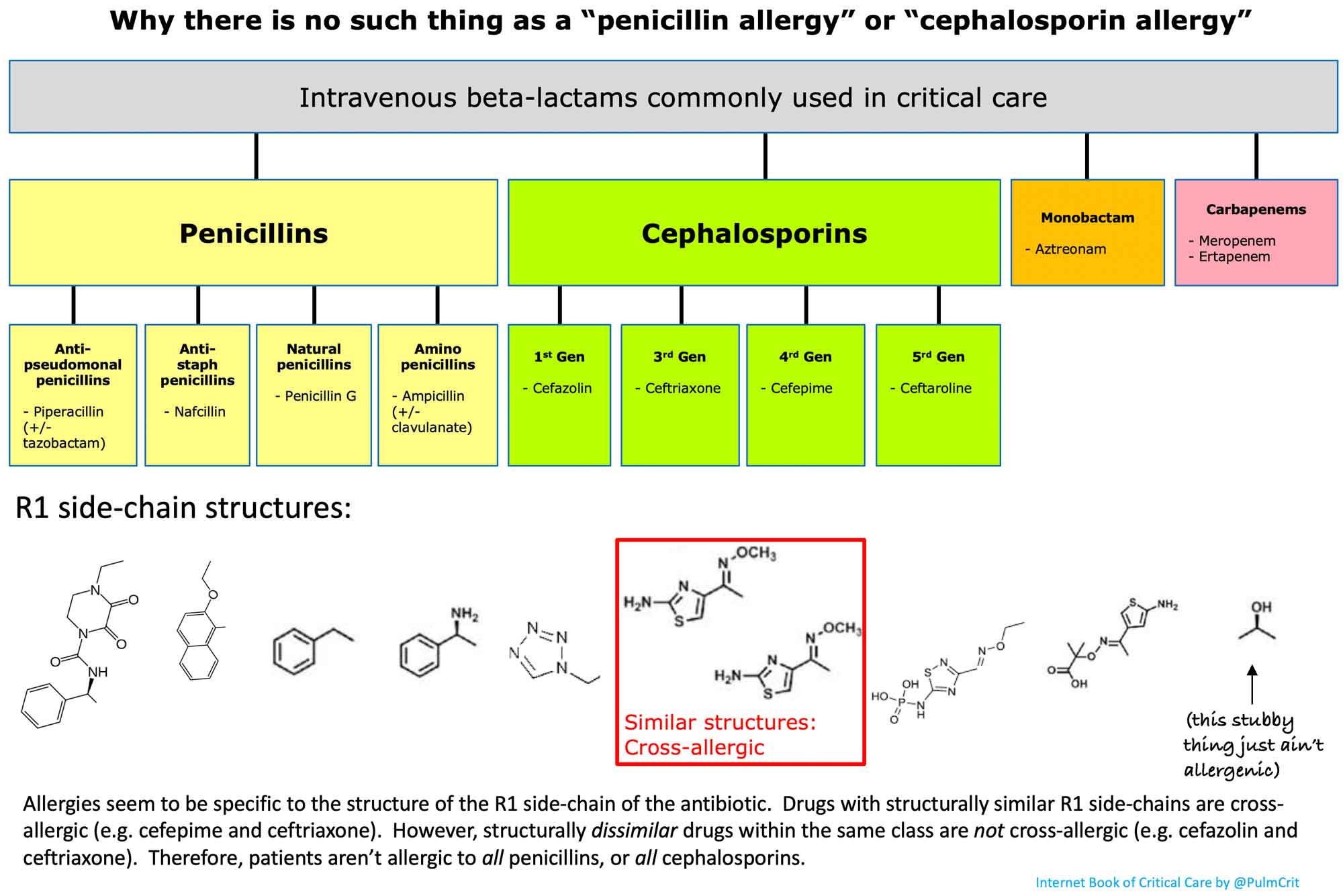 5, Office of the President of the Russian Federation Moscow, Russia, 119121
5, Office of the President of the Russian Federation Moscow, Russia, 119121
Close metadata
According to the recommendations of the European position paper on rhinosinusitis and nasal polyps (EPOS, 2012), the mainstay of treatment for acute bacterial rhinosinusitis (ABRS) is antibacterial drugs [1]. Acute rhinosinusitis is the fifth most commonly prescribed antibiotic in the United States [2]. According to R.F. no such statistics have been published. According to foreign and Russian researchers, the main causative agents of acute bacterial sinusitis are Streptococcus pneumoniae , Haemophilus influenzae producing toxins that block the activity of mucociliary transport and damage the respiratory epithelium, as well as Moraxella 90 030 catarrhalis and Streptococcus pyogenes [3]. Pseudomonas aeruginosa is sown in patients with nosocomial sinusitis, cystic fibrosis, immunodeficiency states, including HIV infection [4, 5]. In addition, recently, in patients with acute bacterial rhinosinusitis without immunological disorders, an increase in St . aureus [6]. In general, in the Russian Federation, ABRS of pneumococcal etiology occurs in 44.9% in all age groups, in 17.3% its cause is Haemophilus influenza (data from a cultural study of the contents of the sinus obtained by aspiration) [7]. This information is of practical value, since in real medical practice in acute rhinosinusitis, the appointment of antibiotic therapy is carried out empirically, due to the fact that culture methods are necessary to determine the pathogen and its sensitivity to antibiotics, and they require a certain amount of time to obtain results. . Therefore, the doctor decides on the appointment of an antibiotic, taking into account the spectrum of action, the level of resistance to this drug, the safety profile, the frequency of administration, the company and the country of manufacture. At the same time, antibiotics are the only class of drugs whose activity decreases over time, and at the same time the number of newly synthesized antibiotics progressively decreases.
In addition, recently, in patients with acute bacterial rhinosinusitis without immunological disorders, an increase in St . aureus [6]. In general, in the Russian Federation, ABRS of pneumococcal etiology occurs in 44.9% in all age groups, in 17.3% its cause is Haemophilus influenza (data from a cultural study of the contents of the sinus obtained by aspiration) [7]. This information is of practical value, since in real medical practice in acute rhinosinusitis, the appointment of antibiotic therapy is carried out empirically, due to the fact that culture methods are necessary to determine the pathogen and its sensitivity to antibiotics, and they require a certain amount of time to obtain results. . Therefore, the doctor decides on the appointment of an antibiotic, taking into account the spectrum of action, the level of resistance to this drug, the safety profile, the frequency of administration, the company and the country of manufacture. At the same time, antibiotics are the only class of drugs whose activity decreases over time, and at the same time the number of newly synthesized antibiotics progressively decreases. The problem of combating antibiotic resistance of bacteria is currently becoming one of the most urgent problems of society. According to the well-known British economist Jim O’Neill, the annual death rate from antibiotic-resistant infections averages 700,000 people. If we extrapolate current trends to 2050, it turns out that by the middle of the century, curable diseases today will claim 10 million human lives. Thus, the rational choice of an antimicrobial drug for empirical therapy is an extremely topical issue: on the one hand, “shooting sparrows from cannons” should be avoided, on the other hand, the possibility of pathogen resistance cannot be ignored. One way to influence the growth of resistance is to avoid the use of the same compounds: the greater the variety of antibiotics that patients use, the more difficult it is for bacteria to develop resistance to them [8]. The second aspect to consider when prescribing antimicrobials is the safety of therapy. Unfortunately, in the pursuit of efficiency, security issues are often overlooked.
The problem of combating antibiotic resistance of bacteria is currently becoming one of the most urgent problems of society. According to the well-known British economist Jim O’Neill, the annual death rate from antibiotic-resistant infections averages 700,000 people. If we extrapolate current trends to 2050, it turns out that by the middle of the century, curable diseases today will claim 10 million human lives. Thus, the rational choice of an antimicrobial drug for empirical therapy is an extremely topical issue: on the one hand, “shooting sparrows from cannons” should be avoided, on the other hand, the possibility of pathogen resistance cannot be ignored. One way to influence the growth of resistance is to avoid the use of the same compounds: the greater the variety of antibiotics that patients use, the more difficult it is for bacteria to develop resistance to them [8]. The second aspect to consider when prescribing antimicrobials is the safety of therapy. Unfortunately, in the pursuit of efficiency, security issues are often overlooked. Below are the main characteristics of the individual classes of drugs recommended for the treatment of ABRS, as well as new data on the safety of cephalosporins in patients allergic to penicillins.
Below are the main characteristics of the individual classes of drugs recommended for the treatment of ABRS, as well as new data on the safety of cephalosporins in patients allergic to penicillins.
In almost all domestic and foreign guidelines, protected penicillins, in particular amoxicillin clavulanate, are considered start drugs in the treatment of acute rhinosinusitis. The main mechanisms of protection of bacteria from this series of drugs are the production of enzymes that destroy the bonds of the β-lactam ring (β-lactamase) and / or modify the antibiotic, and a change in the microorganism cell structure of the target – penicillin-binding proteins (PSB) transpeptidase and carboxypeptidase responsible for the synthesis peptidoglycan of the bacterial cell wall. The addition of clavulanic acid as a β-lactamase inhibitor copes well with the first mechanism, but does not solve the problem with the second, since the mechanism of pneumococcal resistance to penicillin is not associated with the production of β-lactamase, but with the modification of PSB [7].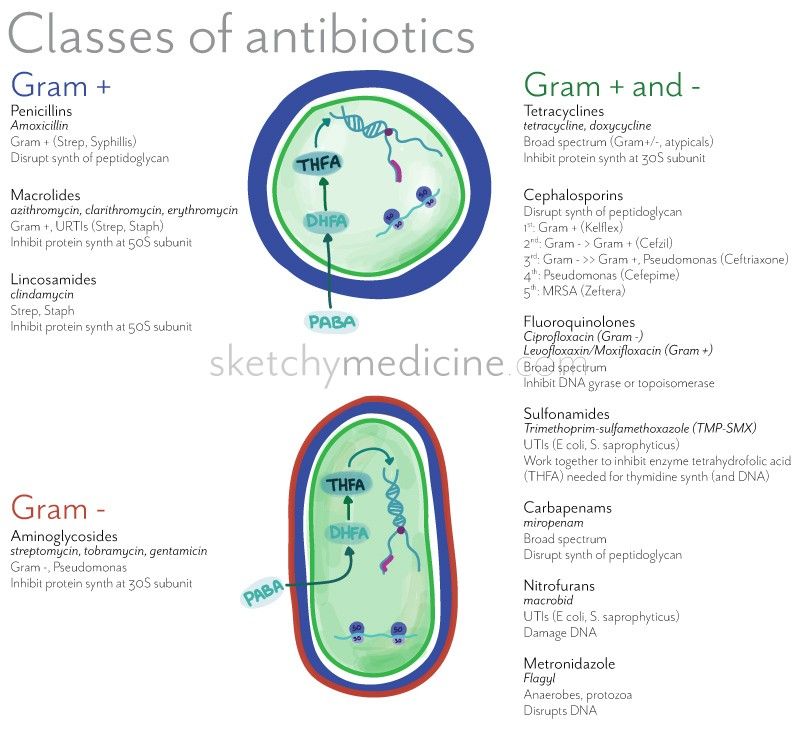 Does this apply to the entire class of β-lactams? Fortunately not: cross-resistance between individual β-lactams is incomplete. A significant proportion of strains Str . pneumoniae , resistant to penicillin, retains sensitivity to third-generation cephalosporins and carbapenems [9]. As for the production of β-lactamase, it should be taken into account that recently there has been an increase in the number of β-lactamase-positive and amoxicillin-clavulanate-resistant strains H . Influenzae (up to 27–43% in the USA) [10–12]. Of the complications when using the combination of amoxicillin + clavulanate, in about 24% of cases there is a diarrheal syndrome associated with the side effects of clavulanic acid due to its incomplete absorption and increased motility of the small intestine [13, 14]. Clavulanate increases the risk of cholestatic or hepatocellular liver damage by 13-23%, which is most often transient and liver function is fully restored after a few weeks.
Does this apply to the entire class of β-lactams? Fortunately not: cross-resistance between individual β-lactams is incomplete. A significant proportion of strains Str . pneumoniae , resistant to penicillin, retains sensitivity to third-generation cephalosporins and carbapenems [9]. As for the production of β-lactamase, it should be taken into account that recently there has been an increase in the number of β-lactamase-positive and amoxicillin-clavulanate-resistant strains H . Influenzae (up to 27–43% in the USA) [10–12]. Of the complications when using the combination of amoxicillin + clavulanate, in about 24% of cases there is a diarrheal syndrome associated with the side effects of clavulanic acid due to its incomplete absorption and increased motility of the small intestine [13, 14]. Clavulanate increases the risk of cholestatic or hepatocellular liver damage by 13-23%, which is most often transient and liver function is fully restored after a few weeks.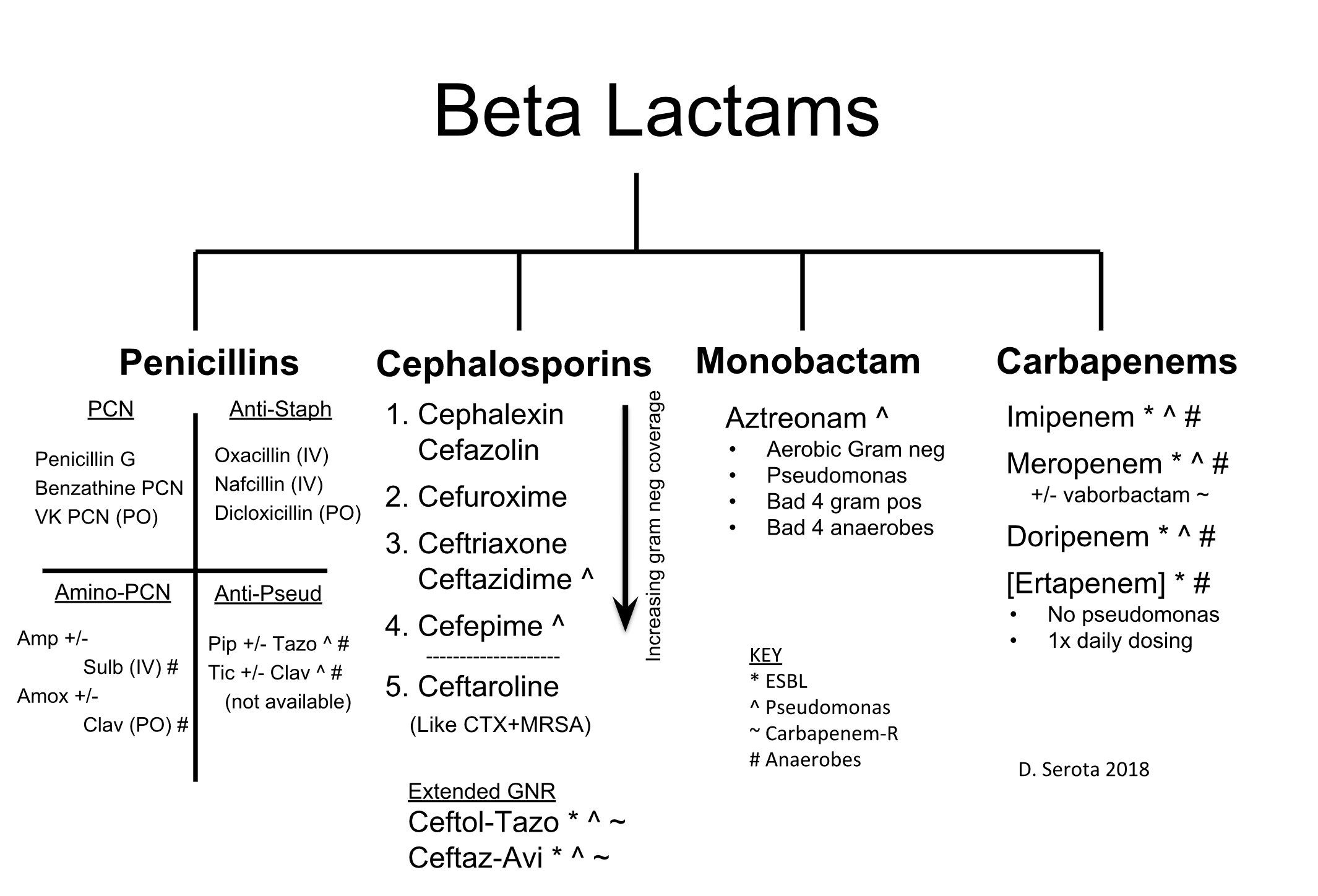 On the other hand, there is a possibility of this complication occurring 8 weeks after the end of the course of antibiotic therapy or an increase in its duration with the development of ductopenia or persistent liver damage [15]. Cholestasis can result from direct effects on hepatocytes or small and large bile ducts [16]. According to British doctors, when taking amoxicillin clavulanate, jaundice was reported in 9.91 cases per 100,000 patients [15].
On the other hand, there is a possibility of this complication occurring 8 weeks after the end of the course of antibiotic therapy or an increase in its duration with the development of ductopenia or persistent liver damage [15]. Cholestasis can result from direct effects on hepatocytes or small and large bile ducts [16]. According to British doctors, when taking amoxicillin clavulanate, jaundice was reported in 9.91 cases per 100,000 patients [15].
Macrolides for acute sinusitis are second-line alternatives and are mainly recommended for β-lactam intolerance. The Infectious Diseases Society of America (IDSA) guidelines for the management of adults and children with ARS do not recommend macrolides (clarithromycin and azithromycin) for empiric therapy due to high rates of resistance in pneumococci (30%). In Asian regions, the situation regarding the decrease in sensitivity to antibiotics of this class is even more sad [12]. In the Russian Federation, according to the latest resistance monitoring data, the level of resistance to azithromycin and clarithromycin exceeded the 20% threshold, which makes it necessary to limit the mass use of macrolides for some time, leaving them for the treatment of chronic rhinosinusitis [19]. The main mechanism for the development of resistance to macrolides is the modification of the target (50S subunit of the bacterial ribosome), while streptococcal methylases are induced by all classes of macrolides, which leads to the development of resistance to all antibiotics of this class. In addition, a special transporter protein of gram-positive bacteria is able to remove 14- and 15-mer macrolides from the cell, although this mechanism is of less clinical significance [9]. Additionally, 14-membered macrolides (including clarithromycin) inhibit the cytochrome-450 system, which leads to the accumulation of toxic metabolites with direct toxic or indirect immunological damage to the liver [20]. Development of cholestatic hepatitis or ductopenia is possible [21]. In addition, it is necessary to remember the cardiotoxicity of macrolides when prescribing them. The arrhythmogenic potential of 14-membered macrolides is higher than that of 15-membered ones [22].
The main mechanism for the development of resistance to macrolides is the modification of the target (50S subunit of the bacterial ribosome), while streptococcal methylases are induced by all classes of macrolides, which leads to the development of resistance to all antibiotics of this class. In addition, a special transporter protein of gram-positive bacteria is able to remove 14- and 15-mer macrolides from the cell, although this mechanism is of less clinical significance [9]. Additionally, 14-membered macrolides (including clarithromycin) inhibit the cytochrome-450 system, which leads to the accumulation of toxic metabolites with direct toxic or indirect immunological damage to the liver [20]. Development of cholestatic hepatitis or ductopenia is possible [21]. In addition, it is necessary to remember the cardiotoxicity of macrolides when prescribing them. The arrhythmogenic potential of 14-membered macrolides is higher than that of 15-membered ones [22].
Respiratory fluoroquinolones (levofloxacin, moxifloxacin) are not recommended for the initial treatment of ABRS, although they are highly active against all pathogens, including pneumococcus, M . catarrhalis and β-lactamase-producing H . influenzae . According to the results of a meta-analysis comparing the effectiveness of respiratory fluoroquinolones and β-lactam antibiotics (amoxicillin clavulanate and third-generation cephalosporins), no significant difference in the timing of regression of clinical symptoms was obtained, but the frequency of side effects with the use of respiratory fluoroquinolones was 2 times higher [23]. The main mechanisms for the development of resistance to quinolones are the modification of the target structure (topoisomerases) and the active removal of the antibiotic from the microbial cell (efflux) by special transport systems [9]. Adverse reactions when taking fluoroquinolones occur in 3-17% of patients [24]. With simultaneous administration of the drug and exposure of the skin to ultraviolet rays, a photosensitivity reaction of varying severity is possible: from slight erythema or vesicles to toxic epidermal necrolysis and skin vasculitis [25].
catarrhalis and β-lactamase-producing H . influenzae . According to the results of a meta-analysis comparing the effectiveness of respiratory fluoroquinolones and β-lactam antibiotics (amoxicillin clavulanate and third-generation cephalosporins), no significant difference in the timing of regression of clinical symptoms was obtained, but the frequency of side effects with the use of respiratory fluoroquinolones was 2 times higher [23]. The main mechanisms for the development of resistance to quinolones are the modification of the target structure (topoisomerases) and the active removal of the antibiotic from the microbial cell (efflux) by special transport systems [9]. Adverse reactions when taking fluoroquinolones occur in 3-17% of patients [24]. With simultaneous administration of the drug and exposure of the skin to ultraviolet rays, a photosensitivity reaction of varying severity is possible: from slight erythema or vesicles to toxic epidermal necrolysis and skin vasculitis [25]. The mechanism of hepatotoxic action of fluoroquinolones is associated with the formation of oxidative radicals in the liver during the metabolism of the drug, which leads to damage to hepatocytes, necrosis and degeneration of the liver tissue; range of lesions – from elevated liver enzymes, jaundice, cholestatic hepatitis to fulminant hepatitis with liver failure [26]. In outpatient practice, the potential neurotoxicity of fluoroquinolones is of primary importance. An indicator of proconvulsant activity, which characterizes the excitatory effect on the central nervous system, exists in all generations of these antibiotics and is manifested by headache, dizziness, and drowsiness [27]. One of the most dangerous side effects associated with taking this group of drugs is rhythm disturbance, including the development of ventricular arrhythmias, and prolongation of the QT interval is currently considered as a group property of fluoroquinolones [24]. Another distinctive feature of this group of drugs is the development of tendinitis at any age and the risk of tendon rupture, most often Achilles, which limits their use in the elderly [27].
The mechanism of hepatotoxic action of fluoroquinolones is associated with the formation of oxidative radicals in the liver during the metabolism of the drug, which leads to damage to hepatocytes, necrosis and degeneration of the liver tissue; range of lesions – from elevated liver enzymes, jaundice, cholestatic hepatitis to fulminant hepatitis with liver failure [26]. In outpatient practice, the potential neurotoxicity of fluoroquinolones is of primary importance. An indicator of proconvulsant activity, which characterizes the excitatory effect on the central nervous system, exists in all generations of these antibiotics and is manifested by headache, dizziness, and drowsiness [27]. One of the most dangerous side effects associated with taking this group of drugs is rhythm disturbance, including the development of ventricular arrhythmias, and prolongation of the QT interval is currently considered as a group property of fluoroquinolones [24]. Another distinctive feature of this group of drugs is the development of tendinitis at any age and the risk of tendon rupture, most often Achilles, which limits their use in the elderly [27]. In addition, fluoroquinolones are contraindicated in pregnancy, lactation, children and adolescents under 18 years of age. In May 2016, the FDA issued a recommendation to limit the use of fluoroquinolones “because the risk of serious side effects generally outweighs the benefits of treatment in patients with acute bacterial sinusitis, exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.” It is recommended that this group of drugs be reserved for use in settings where no alternative treatment or treatment options are available for severe bacterial infections, including anthrax, plague, and bacterial pneumonia, where the benefits of fluoroquinolones outweigh the risk of possible complications.
In addition, fluoroquinolones are contraindicated in pregnancy, lactation, children and adolescents under 18 years of age. In May 2016, the FDA issued a recommendation to limit the use of fluoroquinolones “because the risk of serious side effects generally outweighs the benefits of treatment in patients with acute bacterial sinusitis, exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.” It is recommended that this group of drugs be reserved for use in settings where no alternative treatment or treatment options are available for severe bacterial infections, including anthrax, plague, and bacterial pneumonia, where the benefits of fluoroquinolones outweigh the risk of possible complications.
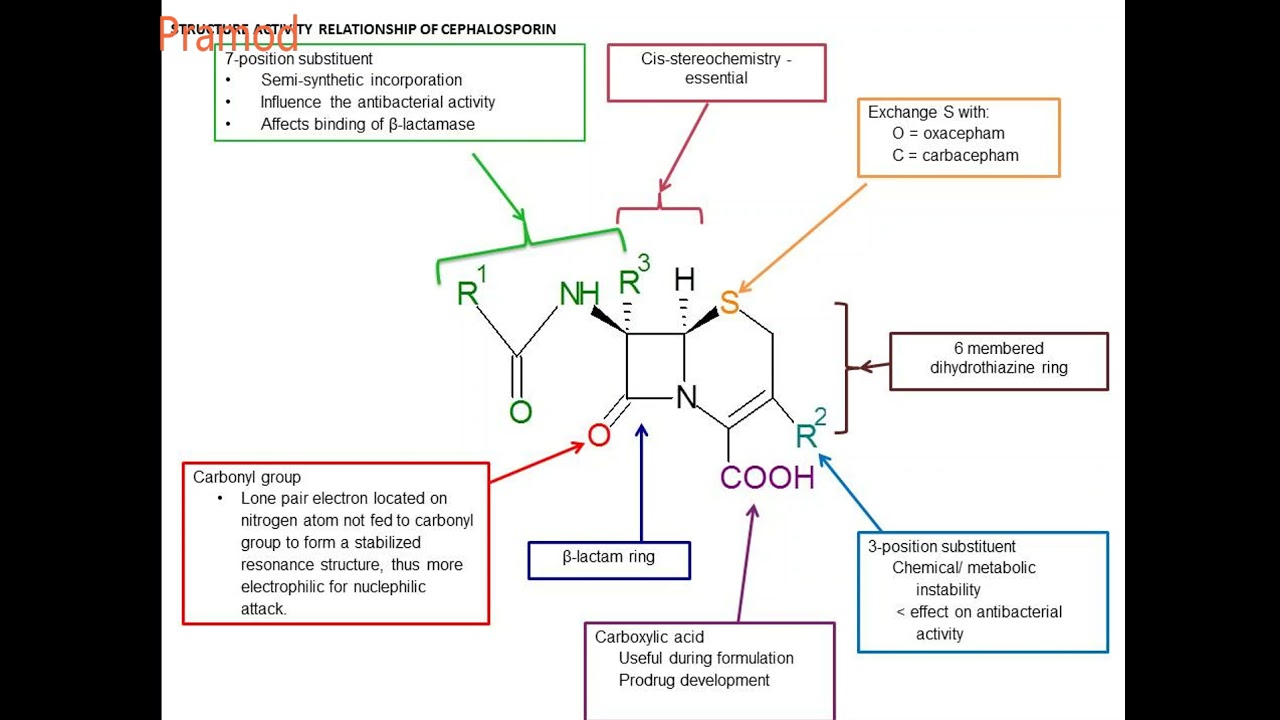
Cephalosporins are β-lactam antibiotics containing, unlike penicillins, dihydrothiazine instead of a thiazolidine ring and two different side chains (R 1 and R 2 ). Chain R 1 is similar to penicillins only in cephalosporins of I and II generations; in cephalosporins of III and IV generations, it is represented by a qualitatively different chemical compound (aminothiazol-oxime group). The variability of the R 2 chain determines the unique pharmacokinetic properties and features of the antibacterial activity of each individual drug in this group. They are well tolerated. The most common side effects when taking them are gastrointestinal disorders (diarrhea, nausea and vomiting). In the guidelines for the treatment of acute inflammatory diseases of the ENT organs, cephalosporins are usually considered as alternative drugs and are not recommended for allergy to β-lactams. The spectrum of action of cephalosporins of different generations is not the same, and even within one III generation there are significant differences between individual representatives; cefixime and ceftibuten have low activity against pneumococcus, which makes it reasonable to use them only in ABRS of hemophilic etiology; ceftriaxone and cefditoren are highly effective against both pathogens. Ceftriaxone has been widely used for a long time, but the availability of only an injectable form limits its use.
The variability of the R 2 chain determines the unique pharmacokinetic properties and features of the antibacterial activity of each individual drug in this group. They are well tolerated. The most common side effects when taking them are gastrointestinal disorders (diarrhea, nausea and vomiting). In the guidelines for the treatment of acute inflammatory diseases of the ENT organs, cephalosporins are usually considered as alternative drugs and are not recommended for allergy to β-lactams. The spectrum of action of cephalosporins of different generations is not the same, and even within one III generation there are significant differences between individual representatives; cefixime and ceftibuten have low activity against pneumococcus, which makes it reasonable to use them only in ABRS of hemophilic etiology; ceftriaxone and cefditoren are highly effective against both pathogens. Ceftriaxone has been widely used for a long time, but the availability of only an injectable form limits its use. Cefditoren is a new oral cephalosporin structurally different from other drugs from the third generation cephalosporin group by the presence of a methylthiazolyl group at the C3 position. This structure of the antibiotic provides increased activity against gram-positive bacteria, including resistance to the pneumococcal penicillin-binding protein PBP2X, which makes it possible to successfully overcome the resistance of penicillin-insensitive strains Streptococcus pneumonia [28].
Cefditoren is a new oral cephalosporin structurally different from other drugs from the third generation cephalosporin group by the presence of a methylthiazolyl group at the C3 position. This structure of the antibiotic provides increased activity against gram-positive bacteria, including resistance to the pneumococcal penicillin-binding protein PBP2X, which makes it possible to successfully overcome the resistance of penicillin-insensitive strains Streptococcus pneumonia [28].
There is a strong opinion in the medical community that in the presence of an allergy to β-lactam antibiotics, cephalosporins should not be prescribed. It is known that the penicillin series leads in the frequency of allergic reactions among all antibacterial drugs (from 15.6 to 54%). Allergic reactions to β-lactam antibiotics arise due to the ability to spontaneously open the β-lactam ring and bind the carbonyl group to the membranes of serum and cellular proteins, forming stable covalent drug-protein adducts known as hapten-carrier conjugates [29]. Initially, there were reports in the literature about the presence of cross-allergy between penicillins and first-generation cephalosporins [30]. During the development of II, III and IV generations of cephalosporins, they were modified in terms of the size and complexity of their side chains, which was manifested by a change in cross-reactivity between penicillins and cephalosporins. As noted by M.E. Pichichero [31], the level of tolerance between these classes of antibiotics is 98%, which is possibly lower than between penicillins and other antibacterial drugs. The risk of anaphylactic reactions to cephalosporins is estimated at 0.0001–0.1%, and there is no proven correlation between groups of patients with and without penicillin allergy [32]. Therefore, a ban on the use of the entire group of cephalosporins in the presence of a history of allergy to penicillins may have more negative consequences than positive ones. According to M.E. Pichichero [31], the use of III—IV generation cephalosporins, whose side chains differ from penicillin, for penicillin allergy is legally justified and substantiated from the standpoint of evidence-based medicine.
Initially, there were reports in the literature about the presence of cross-allergy between penicillins and first-generation cephalosporins [30]. During the development of II, III and IV generations of cephalosporins, they were modified in terms of the size and complexity of their side chains, which was manifested by a change in cross-reactivity between penicillins and cephalosporins. As noted by M.E. Pichichero [31], the level of tolerance between these classes of antibiotics is 98%, which is possibly lower than between penicillins and other antibacterial drugs. The risk of anaphylactic reactions to cephalosporins is estimated at 0.0001–0.1%, and there is no proven correlation between groups of patients with and without penicillin allergy [32]. Therefore, a ban on the use of the entire group of cephalosporins in the presence of a history of allergy to penicillins may have more negative consequences than positive ones. According to M.E. Pichichero [31], the use of III—IV generation cephalosporins, whose side chains differ from penicillin, for penicillin allergy is legally justified and substantiated from the standpoint of evidence-based medicine. The American Academy of Pediatrics and American Academy of Family Physicians guidelines for the treatment of acute otitis media in children directly indicate the use of third-generation cephalosporins despite a history of penicillin allergy [33].
The American Academy of Pediatrics and American Academy of Family Physicians guidelines for the treatment of acute otitis media in children directly indicate the use of third-generation cephalosporins despite a history of penicillin allergy [33].
In summary, it should be noted that at present the main treatment for acute bacterial rhinosinusitis is antibiotic therapy. Each group of antibiotics has its own indications and contraindications, as well as side effects. Of course, the doctor should always take into account the potential risk of developing an allergic reaction and, when prescribing an antibiotic, follow the instructions for its use. It is gratifying to note that new antibacterial drugs appear on the domestic market, in particular the antibiotic Spectracef. Many clinicians still do not have experience with the use of this drug in clinical work. That is why the initiated study is relevant and its results will serve the correct use of Spectracef in clinical practice.
The aim of the study was to evaluate the efficacy and safety of Spectracef in the treatment of acute bacterial rhinosinusitis in outpatient practice.
Patients and Methods
Under observation were 30 patients with a diagnosis of acute purulent rhinosinusitis, aged 19 to 67 years, including 11 women and 19 men. The study was conducted in the department of otorhinolaryngology of the Federal State Budgetary Institution “Polyclinic No. 5” of the Office of the President R.F. in the period from March to September 2016. The diagnosis was established on the basis of the clinical picture, endoscopic examination data and radiography of the paranasal sinuses. When making a diagnosis, clinical symptoms were taken into account: complaints of headache, heaviness in the projection of the paranasal sinuses, difficulty in nasal breathing, nasal discharge of a mucopurulent nature, weakness, low-grade body temperature. When analyzing radiographs, a decrease in the pneumatization of the paranasal sinuses was noted in the form of parietal edema and a total decrease in their airiness.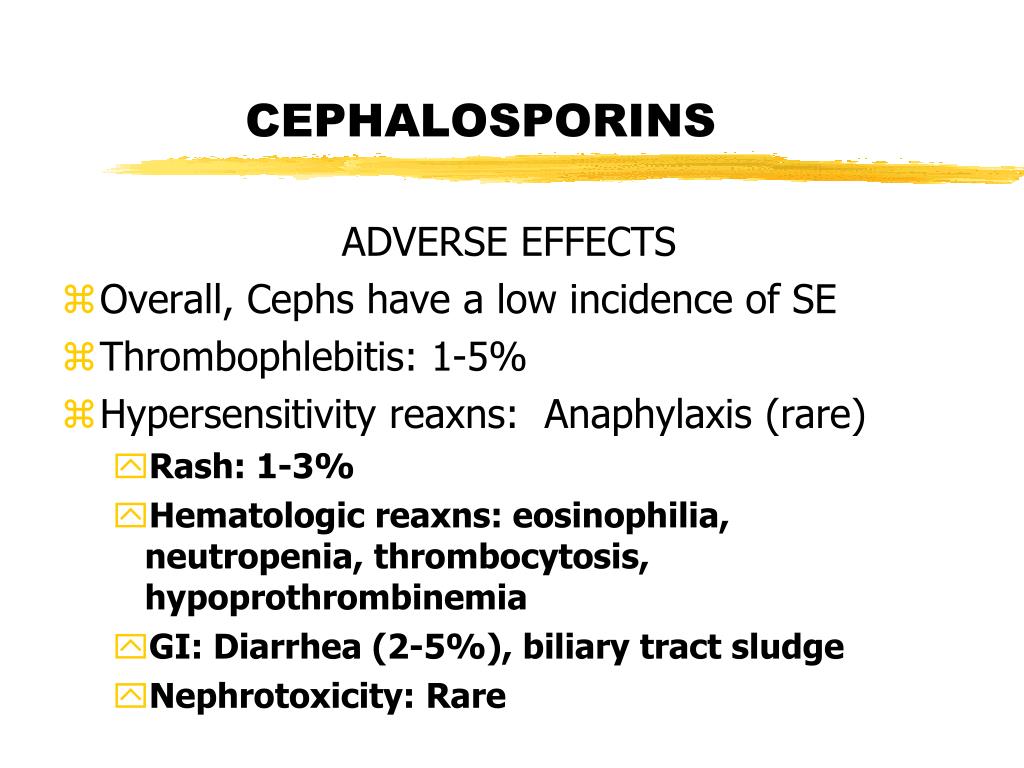 The maxillary and ethmoid sinuses were more often affected, less frequently the frontal ones. In 18 (60%) patients, the process was bilateral. All patients noted ARVI transferred 5-15 days ago. Some patients noted a sudden deterioration in well-being a few days after the apparent positive dynamics of the course of acute respiratory viral infections with a tendency to recovery. Other patients noted a gradual deterioration in well-being against the background of SARS. The duration of the current illness (rhinosinusitis) before seeking medical help ranged from 1 to 8 days. The exclusion criterion was treatment with antibacterial drugs for 2 months before the current visit to the otorhinolaryngologist, the presence of chronic rhinosinusitis.
The maxillary and ethmoid sinuses were more often affected, less frequently the frontal ones. In 18 (60%) patients, the process was bilateral. All patients noted ARVI transferred 5-15 days ago. Some patients noted a sudden deterioration in well-being a few days after the apparent positive dynamics of the course of acute respiratory viral infections with a tendency to recovery. Other patients noted a gradual deterioration in well-being against the background of SARS. The duration of the current illness (rhinosinusitis) before seeking medical help ranged from 1 to 8 days. The exclusion criterion was treatment with antibacterial drugs for 2 months before the current visit to the otorhinolaryngologist, the presence of chronic rhinosinusitis.
All patients were prescribed Spectracef (cefditoren) 200 mg twice daily for 10 days, nasal irrigation and decongestants. The evaluation of the clinical efficacy of the study drug was carried out on the basis of the dynamics of clinical symptoms. Before treatment, on days 5 and 10, patients assessed the following symptoms using a 10-point visual analog scale (VAS): headache, nasal congestion, nasal discharge. At the same time, during endoscopic examination, the condition of the nasal mucosa was assessed, and the presence of a pathological secretion was noted. For statistical data analysis, the AtteStat program, version 2.8.0, was used. Due to the rejection of the hypothesis of normal distribution for all characteristics, the indicators were calculated as a median (M) and interquartile intervals of the 25th and 75th intervals [in square brackets].
Before treatment, on days 5 and 10, patients assessed the following symptoms using a 10-point visual analog scale (VAS): headache, nasal congestion, nasal discharge. At the same time, during endoscopic examination, the condition of the nasal mucosa was assessed, and the presence of a pathological secretion was noted. For statistical data analysis, the AtteStat program, version 2.8.0, was used. Due to the rejection of the hypothesis of normal distribution for all characteristics, the indicators were calculated as a median (M) and interquartile intervals of the 25th and 75th intervals [in square brackets].
Results and discussion
An analysis of the results of treatment showed a positive trend in the relief of all the studied symptoms of the disease (see figure). Headache on day 5 persisted only in 30% of cases, its intensity according to VAS significantly decreased: 6 [3–9] before treatment, 0 [0–1] on day 5. On the 10th day of illness, none of the patients noted this symptom.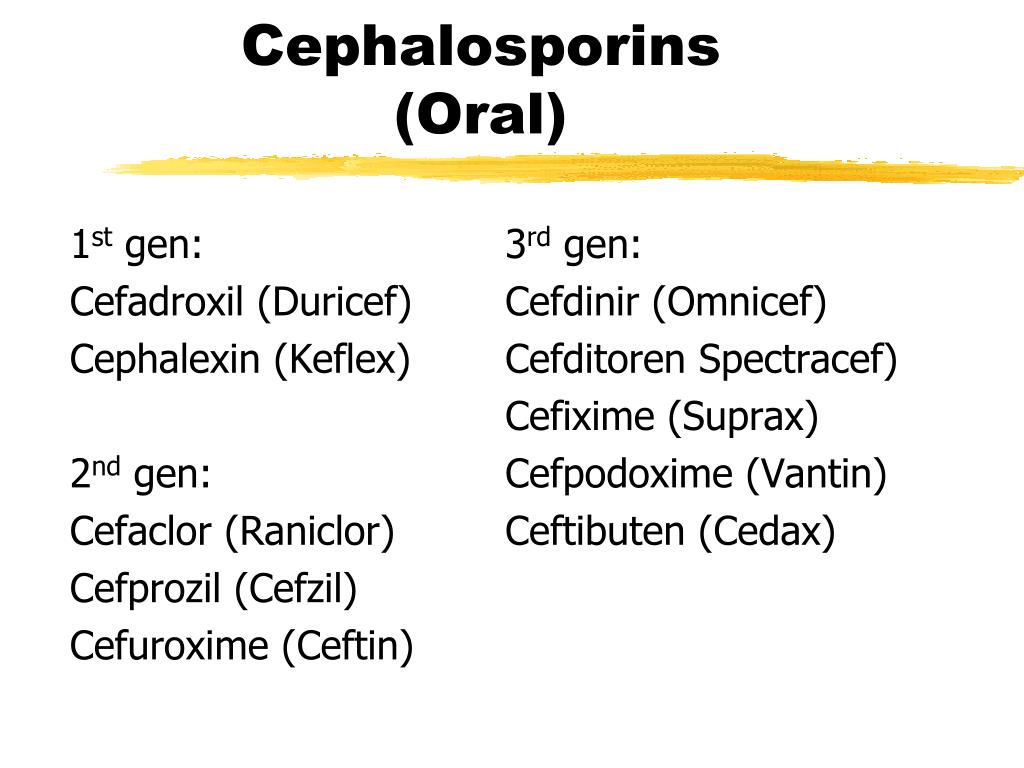 The severity of nasal discharge also significantly decreased at the second visit on the 5th day of illness (6 [6—9] and 3 [3-6]), on examination, the nature of the discharge changed from purulent to mucous. On day 10, 5 (30%) patients had mild seromucosal discharge (median 0 [0–1]). A rather unpleasant symptom of nasal congestion, as well as rhinorrhea, was present in 100% of cases (median 6 [3–9]). Upon re-examination on the 5th day, patients noted a significant improvement in nasal breathing (M=3 [3–4]). On day 10, minor nasal congestion persisted in 20% of cases (median 0 [0–1]), but in our clinical observation, patients took only decongestants and irrigation therapy preparations (saline, sea water) from local agents. The addition of topical corticosteroids to the treatment would greatly accelerate the regression of the symptom of nasal congestion. When evaluating the safety of the antibacterial drug, it can be noted that Spectracef (cefditoren) is well tolerated: there were no complaints about the development of adverse drug reactions, including those from the gastrointestinal tract.
The severity of nasal discharge also significantly decreased at the second visit on the 5th day of illness (6 [6—9] and 3 [3-6]), on examination, the nature of the discharge changed from purulent to mucous. On day 10, 5 (30%) patients had mild seromucosal discharge (median 0 [0–1]). A rather unpleasant symptom of nasal congestion, as well as rhinorrhea, was present in 100% of cases (median 6 [3–9]). Upon re-examination on the 5th day, patients noted a significant improvement in nasal breathing (M=3 [3–4]). On day 10, minor nasal congestion persisted in 20% of cases (median 0 [0–1]), but in our clinical observation, patients took only decongestants and irrigation therapy preparations (saline, sea water) from local agents. The addition of topical corticosteroids to the treatment would greatly accelerate the regression of the symptom of nasal congestion. When evaluating the safety of the antibacterial drug, it can be noted that Spectracef (cefditoren) is well tolerated: there were no complaints about the development of adverse drug reactions, including those from the gastrointestinal tract.

 Zhonghua Min Guo Wei Sheng Wu Xue Za Zhi. 1975 Mar;8(1):1-11. [PubMed: 1097210]
Zhonghua Min Guo Wei Sheng Wu Xue Za Zhi. 1975 Mar;8(1):1-11. [PubMed: 1097210] Ceftaroline: a novel broad-spectrum cephalosporin with activity against meticillin-resistant Staphylococcus aureus. Drugs. 2009;69(7):809-31. [PubMed: 19441869]
Ceftaroline: a novel broad-spectrum cephalosporin with activity against meticillin-resistant Staphylococcus aureus. Drugs. 2009;69(7):809-31. [PubMed: 19441869] 2008 May;7(3):295-304. [PubMed: 18462187]
2008 May;7(3):295-304. [PubMed: 18462187] 1989 Summer;8(2):73-88. [PubMed: 2672726]
1989 Summer;8(2):73-88. [PubMed: 2672726] Pharmacotherapy. 2005 Oct;25(10):1389-95. [PubMed: 16185184]
Pharmacotherapy. 2005 Oct;25(10):1389-95. [PubMed: 16185184]
 But they’re a little less effective against Gram-positive bacteria compared to first-generation cephalosporins
But they’re a little less effective against Gram-positive bacteria compared to first-generation cephalosporins
 Seek immediate medical treatment if you’re taking a cephalosporin and experience symptoms of anaphylaxis.
Seek immediate medical treatment if you’re taking a cephalosporin and experience symptoms of anaphylaxis.