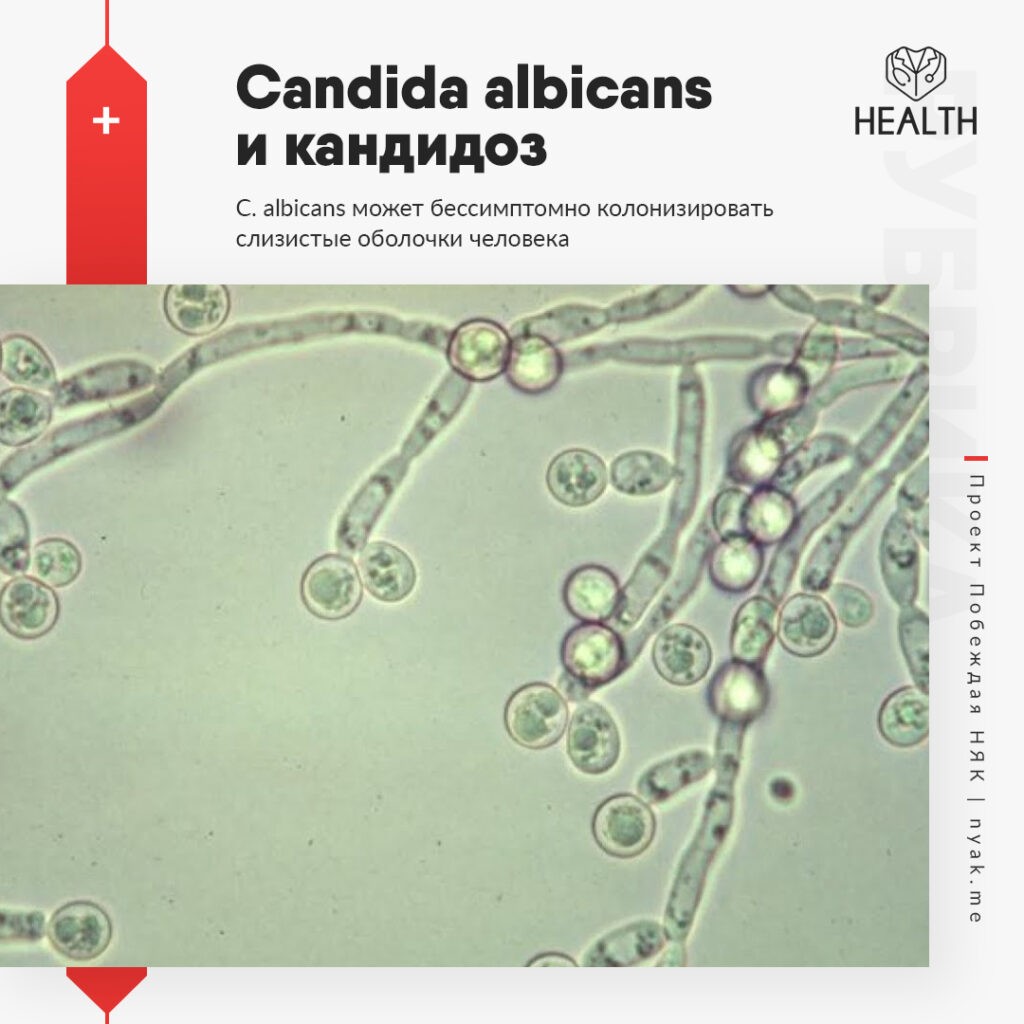
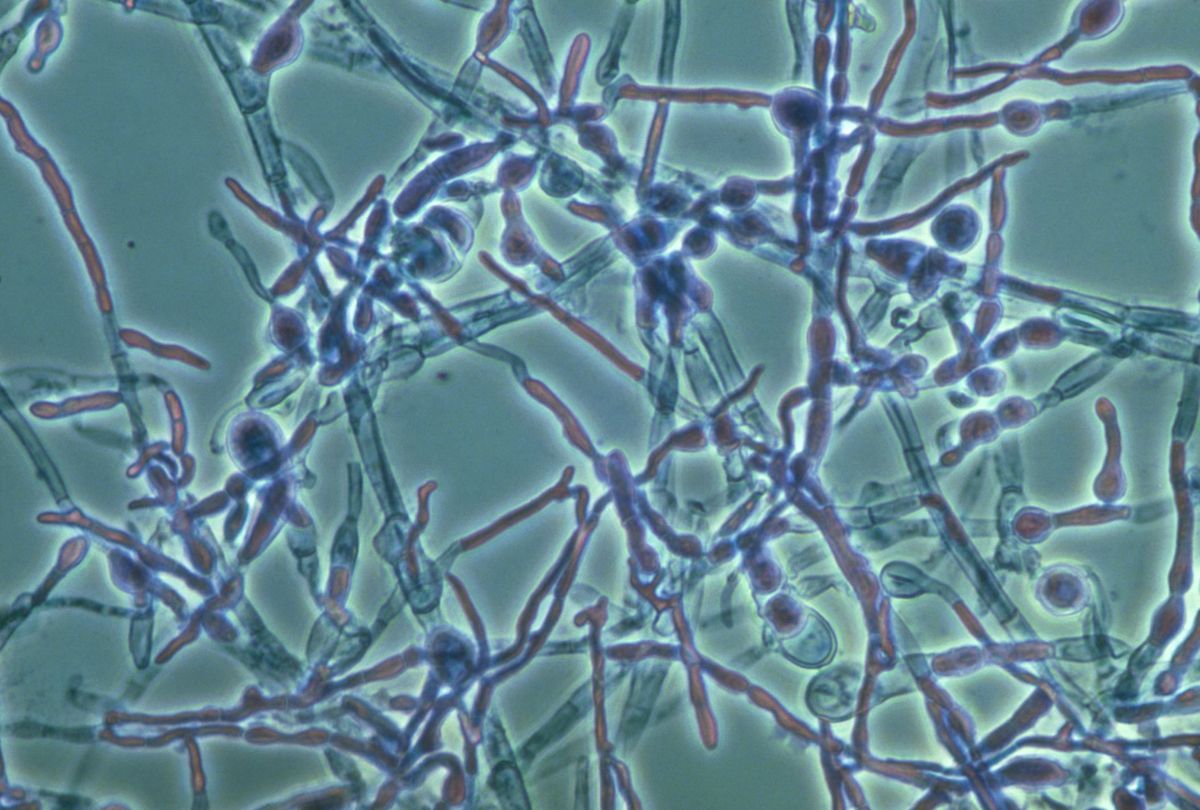
Picture of candida albicans. Candida Albicans: The Shape-Shifting Fungus and Its Role in Infections
How does Candida albicans switch between yeast and hyphal forms. What role does the Sir2 protein play in this transition. How does nutrient availability affect C. albicans morphology. Why is understanding C. albicans’ shape-shifting ability important for medical research.
The Dual Nature of Candida Albicans: Yeast and Hypha Forms
Candida albicans is a remarkable fungus known for its ability to shift between two distinct forms: single, oval-shaped cells called yeast and elongated, thread-like filaments known as hyphae. This shape-shifting capability plays a crucial role in the pathogen’s survival within the human body and its potential to cause various infections, including dangerous hospital-acquired ones.
Understanding the mechanisms behind C. albicans’ morphological transitions is essential for developing effective treatments against this opportunistic pathogen. Recent research has shed light on one of the key factors involved in this process: a protein called Sir2.

The Importance of Morphological Transitions
Why is C. albicans’ ability to switch between forms so significant? Both the yeast and hyphal forms serve distinct purposes during infection:
- The yeast form allows for rapid multiplication and dissemination within the host.
- The hyphal form enables the fungus to invade tissues and evade the immune system more effectively.
This versatility makes C. albicans a formidable pathogen, capable of adapting to different environments within the human body and causing a wide range of infections, from superficial skin conditions to life-threatening systemic candidiasis.
The Role of Sir2 in Candida Albicans Morphology
Recent research conducted by University at Buffalo biologists Guolei Zhao and Laura Rusche has unveiled the significant role of the Sir2 protein in C. albicans’ morphological transitions. Their study, published in the journal mSphere, provides valuable insights into the molecular mechanisms underlying this shape-shifting ability.
Key Findings of the Sir2 Study
What did the researchers discover about Sir2’s influence on C. albicans morphology?
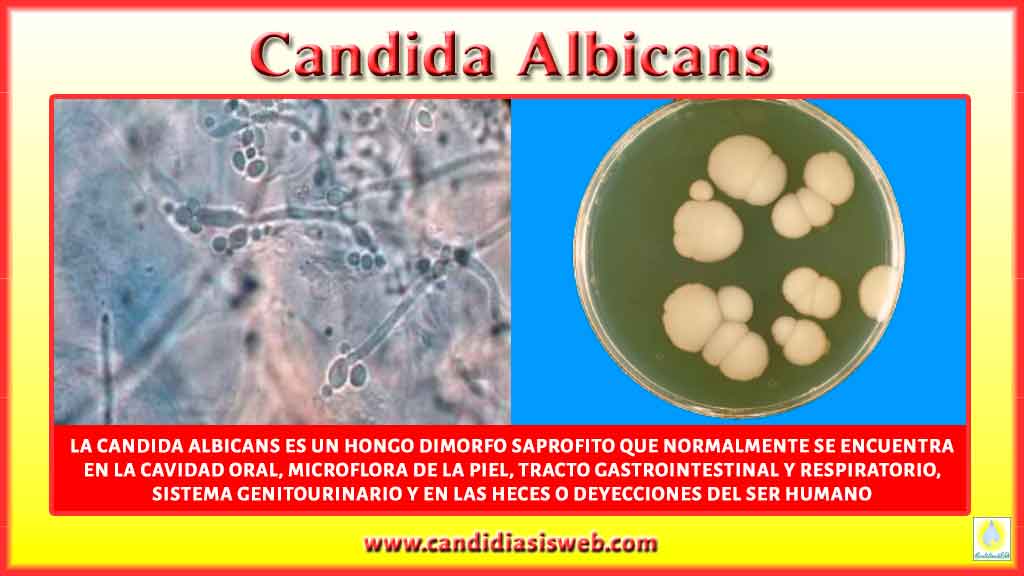
- C. albicans cells lacking the Sir2 gene were less likely to form true hyphae in laboratory experiments.
- The protein’s impact on morphology varied depending on the nutrient availability in the environment.
- Sir2 appears to be involved in helping C. albicans cells interpret and respond to external cues.
These findings suggest that Sir2 plays a crucial role in the complex decision-making process that determines whether C. albicans takes on its yeast or filamentous form.
Nutrient Availability and Its Impact on C. Albicans Morphology
The researchers observed intriguing differences in C. albicans’ behavior depending on the nutrient content of its environment. How does nutrient availability affect the fungus’ morphology?
- In nutrient-poor conditions:
- Cells lacking Sir2 were less likely to form both true hyphae and pseudohyphae (an intermediate form).
- In nutrient-rich conditions:
- Cells without Sir2 formed more pseudohyphae but fewer true hyphae.
These observations highlight the complex interplay between environmental factors and genetic regulation in determining C. albicans’ morphology. The Sir2 protein appears to be a key player in integrating these various signals and influencing the fungus’ form.

Molecular Mechanisms of Sir2’s Influence on Hyphal Formation
To gain a deeper understanding of how Sir2 affects hyphal formation, the researchers investigated various aspects of C. albicans biology. What did they discover about the molecular mechanisms involved?
- Sir2 protein localization:
- The Sir2 protein was found to be localized in the nucleus of C. albicans cells.
- Gene activity changes:
- Removing the Sir2 gene led to decreased activity of certain genes typically highly active in hyphal cells.
- Deacetylation function:
- The Sir2 protein’s ability to remove acetyl groups from other proteins appears to be crucial for facilitating the transition to hyphae.
These findings provide valuable insights into the molecular pathways involved in C. albicans’ morphological transitions and highlight potential targets for future antifungal therapies.
Implications for Medical Research and Antifungal Treatments
Understanding the mechanisms behind C. albicans’ shape-shifting ability has significant implications for medical research and the development of new antifungal treatments. How might these findings be applied in the fight against C. albicans infections?
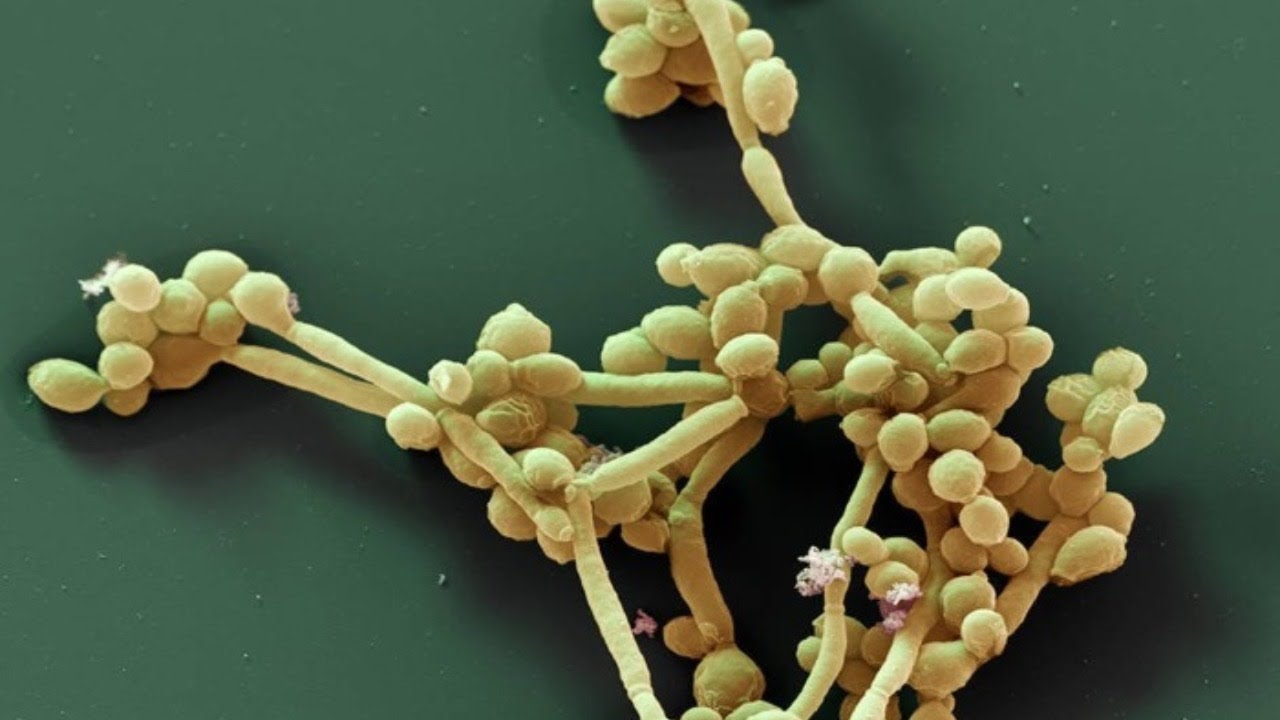
- Targeted therapies:
- Developing drugs that interfere with Sir2’s function or other proteins involved in the yeast-to-hypha transition could potentially reduce C. albicans’ virulence.
- Biofilm prevention:
- Understanding the factors that influence hyphal formation may lead to strategies for preventing or disrupting C. albicans biofilms, which are notoriously difficult to treat.
- Improved diagnostics:
- Knowledge of the molecular mechanisms involved in morphological transitions could lead to more accurate and rapid diagnostic tools for C. albicans infections.
By elucidating the role of Sir2 and other factors in C. albicans’ shape-shifting ability, researchers are paving the way for novel approaches to combat this resilient pathogen.
The Broader Context: Candida Species and Hospital-Acquired Infections
While C. albicans is a significant concern in healthcare settings, it is not the only Candida species causing problems. The emergence of drug-resistant strains, such as Candida auris, has further complicated the landscape of fungal infections in hospitals.
The Rise of Candida Auris
Candida auris, a relatively newly discovered species, has gained notoriety for its ability to cause severe infections and resist multiple antifungal drugs. How does C. auris compare to C. albicans in terms of hospital-acquired infections?
- Discovery:
- C. auris was first identified in 2009, making it a relatively new threat compared to the well-studied C. albicans.
- Drug resistance:
- C. auris is often resistant to multiple classes of antifungal drugs, making it particularly challenging to treat.
- Environmental persistence:
- C. auris can survive on surfaces for extended periods, making it difficult to eradicate from healthcare settings.
The emergence of C. auris highlights the importance of ongoing research into fungal pathogens and the need for new approaches to prevent and treat hospital-acquired infections.
Future Directions in Candida Research
The study of C. albicans’ shape-shifting ability and the role of proteins like Sir2 opens up new avenues for research in the field of medical mycology. What are some potential areas of focus for future studies?
- Comprehensive understanding of morphological regulators:
- Investigating other proteins and genetic factors involved in C. albicans’ yeast-to-hypha transition.
- Environmental sensing mechanisms:
- Elucidating how C. albicans interprets various environmental cues to trigger morphological changes.
- Comparative studies with other Candida species:
- Exploring similarities and differences in morphological regulation across different Candida species, including emerging threats like C. auris.
- Host-pathogen interactions:
- Investigating how C. albicans’ different morphological forms interact with the host immune system and influence infection outcomes.
- Novel antifungal strategies:
- Developing innovative approaches to target the morphological transitions of Candida species as a means of preventing or treating infections.
By pursuing these and other research directions, scientists can continue to unravel the complexities of Candida biology and develop more effective strategies for combating these persistent pathogens.

The Importance of Interdisciplinary Approaches in Fungal Research
The study of Candida albicans and other fungal pathogens requires a multidisciplinary approach, bringing together experts from various fields. How can interdisciplinary collaboration enhance our understanding of these organisms and lead to better treatment options?
- Molecular biology and genetics:
- Unraveling the genetic basis of morphological transitions and virulence factors.
- Biochemistry:
- Studying the structure and function of proteins like Sir2 and their role in cellular processes.
- Microbiology:
- Investigating the growth patterns and behaviors of Candida species in various environments.
- Immunology:
- Examining host-pathogen interactions and the immune response to Candida infections.
- Pharmacology:
- Developing and testing new antifungal compounds targeting specific aspects of Candida biology.
- Clinical medicine:
- Translating laboratory findings into practical applications for diagnosing and treating Candida infections.
By fostering collaboration between these diverse fields, researchers can gain a more comprehensive understanding of Candida species and develop innovative solutions to combat fungal infections.

The Role of Advanced Technologies in Candida Research
Cutting-edge technologies are playing an increasingly important role in advancing our understanding of Candida biology and pathogenesis. How are these technologies contributing to fungal research?
- Next-generation sequencing:
- Enabling rapid and comprehensive genetic analysis of Candida strains and their evolution.
- CRISPR-Cas9 gene editing:
- Allowing precise genetic modifications to study gene function and create model organisms.
- Advanced microscopy techniques:
- Providing high-resolution imaging of Candida morphology and host-pathogen interactions.
- Proteomics and metabolomics:
- Offering insights into the complex molecular processes occurring during morphological transitions and infection.
- Artificial intelligence and machine learning:
- Analyzing large datasets to identify patterns and predict fungal behavior or drug responses.
These advanced technologies are accelerating the pace of discovery in Candida research and opening up new possibilities for understanding and combating these resilient pathogens.
The Global Impact of Candida Infections and Research Initiatives
Candida infections, including those caused by C. albicans and emerging threats like C. auris, have a significant global impact on public health. How are different countries and international organizations addressing this challenge?
- Surveillance programs:
- Implementing global monitoring systems to track the spread of drug-resistant Candida strains.
- Research collaborations:
- Fostering international partnerships to share knowledge and resources in the fight against fungal pathogens.
- Policy initiatives:
- Developing guidelines and policies for fungal infection prevention and control in healthcare settings.
- Education and awareness:
- Promoting public understanding of fungal infections and the importance of antifungal stewardship.
- Funding priorities:
- Allocating resources to support research into novel antifungal therapies and diagnostic tools.
By coordinating efforts on a global scale, the scientific community can more effectively address the challenges posed by Candida infections and work towards better prevention and treatment strategies.
The Role of Candida Research in One Health Initiatives
The study of Candida species and their interactions with humans and the environment aligns with the One Health approach, which recognizes the interconnectedness of human, animal, and environmental health. How does Candida research contribute to this holistic perspective?
- Ecological studies:
- Investigating the natural habitats and reservoirs of Candida species in the environment.
- Zoonotic potential:
- Exploring the possibility of Candida transmission between animals and humans.
- Agricultural impacts:
- Studying the effects of Candida species on crop plants and livestock.
- Environmental factors:
- Examining how climate change and other environmental shifts may influence the distribution and behavior of Candida species.
- Antifungal resistance:
- Investigating the development and spread of antifungal resistance in both clinical and environmental settings.
By adopting a One Health perspective, researchers can gain a more comprehensive understanding of Candida ecology and epidemiology, leading to more effective strategies for managing these pathogens across different domains.

From yeast to hypha: How Candida albicans makes the switch
C. albicans’ ability to shift forms can help it cause dangerous infections, and a study suggests that a protein called Sir2 aids this transition
BUFFALO, N.Y. — You might call Candida albicans a shape-shifter: As this fungus grows, it can multiply as single, oval-shaped cells called yeast or propagate in an elongated form called hypha, consisting of thread-like filaments.
This dual nature can help the pathogen survive in the body, where it can cause disease, including dangerous hospital-acquired infections.
But how does this switching ability occur?
New research identifies one factor that may contribute. In a study published on May 5 in the journal mSphere, University at Buffalo biologists Guolei Zhao and Laura Rusche report that a protein called Sir2 may facilitate C. albicans’ transition from ovoid yeast to thread-like hypha. C. albicans cells that were missing the Sir2 gene were less likely to form true hyphae in lab experiments than cells of the same species that had that gene.
albicans cells that were missing the Sir2 gene were less likely to form true hyphae in lab experiments than cells of the same species that had that gene.
“When we got rid of the Sir2 gene, we saw less of the true hyphae form,” says Zhao, first author and a PhD candidate in biological sciences in the UB College of Arts and Sciences. This is interesting, she says, because both the “tiny round yeast form” and the “elongated hyphae form” are “essential to infection,” helping C. albicans invade different niches of the human body.
The influence of Sir2 on morphology differed depending on the cells’ surroundings: In a nutrient-poor environment, C. albicans cells that were missing the Sir2 gene were less likely to form both true hyphae and pseudohyphae, a sort of in-between stage where the cells are elongated and grow in chains. But in a nutrient-rich situation, C. albicans lacking the Sir2 gene formed more pseudohyphae even as the formation of true hyphae declined.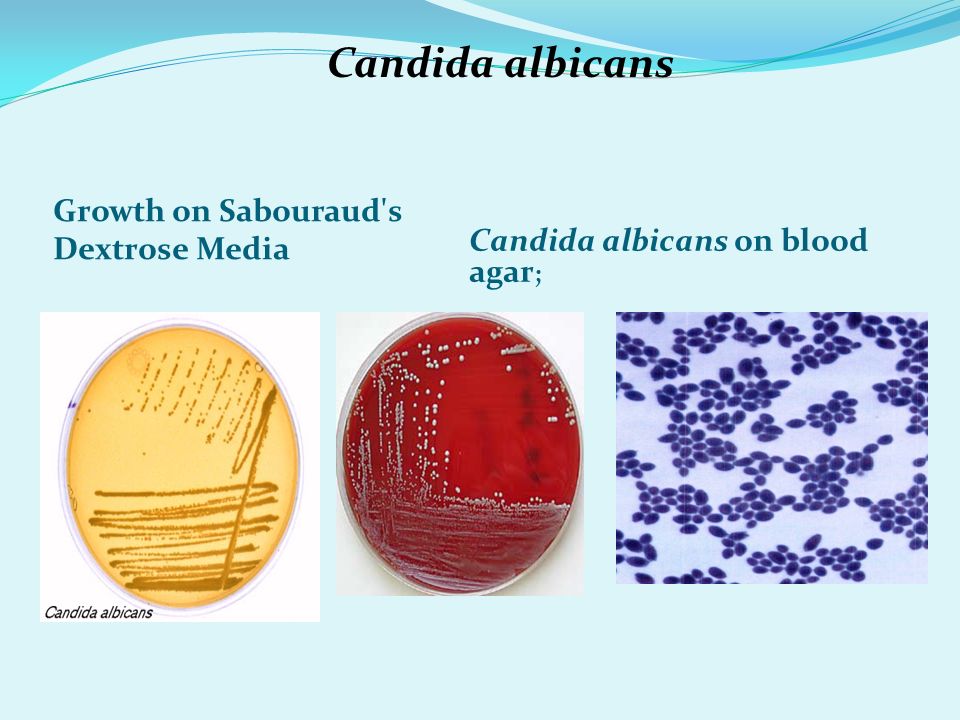
More research is needed to understand why, exactly, this might happen, but, “Clearly, the nutrient environment changes the behavior of the cells,” says Rusche, PhD, UB associate professor of biological sciences.
Rusche explains that different cues in the environment — such as the availability of nutrients and temperature — influence whether C. albicans takes on a yeast or filamentous form. Sir2, which belongs to a family of proteins called sirtuins, may impact this process, helping cells interpret and/or respond to what is happening in the external world, she says.
“From our perspective, what’s important is that in either condition — nutrient-rich or nutrient-poor — not having the Sir2 gene changed the balance, which implies that the signal the Sir2 protein is transmitting is an important part of the equation,” Rusche says. “Cells are integrating a lot of information to ‘decide’ what form to take. Knowing more about what triggers the choice could allow us to modulate it in the future.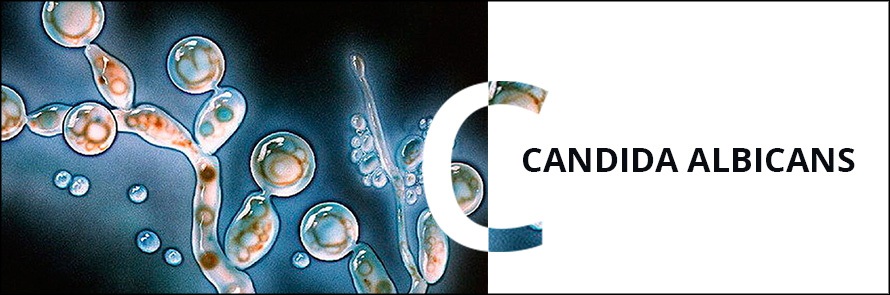 If you can reduce Candida albicans’ ability to generate the filamentous form, maybe you can make it less infectious.”
If you can reduce Candida albicans’ ability to generate the filamentous form, maybe you can make it less infectious.”
To learn more about how Sir2 might impact hyphal formation, Rusche and Zhao took a closer look at various aspects of C. albicans’ biology. Among other findings, the scientists showed that the Sir2 protein is localized in the nucleus of C. albicans’ cells, and that removing the Sir2 gene from C. albicans led to a decrease in the activity of certain genes that are usually highly active in hyphal cells.
The researchers also concluded that one of the Sir2 protein’s key functions — removing an acetyl group from other proteins — is likely involved in facilitating the transition to hyphae. In experiments, disrupting this process of deacetylation resulted in fewer true hyphae being formed.
“Sir2 is a protein that I’ve been studying for 20 years, so it’s been at the center of my research for a long time,” Rusche says. “We’ve been interested in how it has different functions in different species. We decided it would be interesting to look at the Sir2 protein in Candida albicans because it has medical relevance, and we wanted to see what our knowledge about this protein can help us learn about the pathogen.”
We decided it would be interesting to look at the Sir2 protein in Candida albicans because it has medical relevance, and we wanted to see what our knowledge about this protein can help us learn about the pathogen.”
“I’m interested in pathogenic species,” Zhao says. “The Sir2 protein seems to have this effect in the morphological transition between yeast and hyphal form. We think that this transition may impact the virulence of this human pathogen, so that’s very important.”
The research was supported by a pilot project award from the UB Genome, Environment and Microbiome Community of Excellence.
Candida auris: What is the deadly fungus sweeping through US hospitals?
Published
Image source, Getty Images
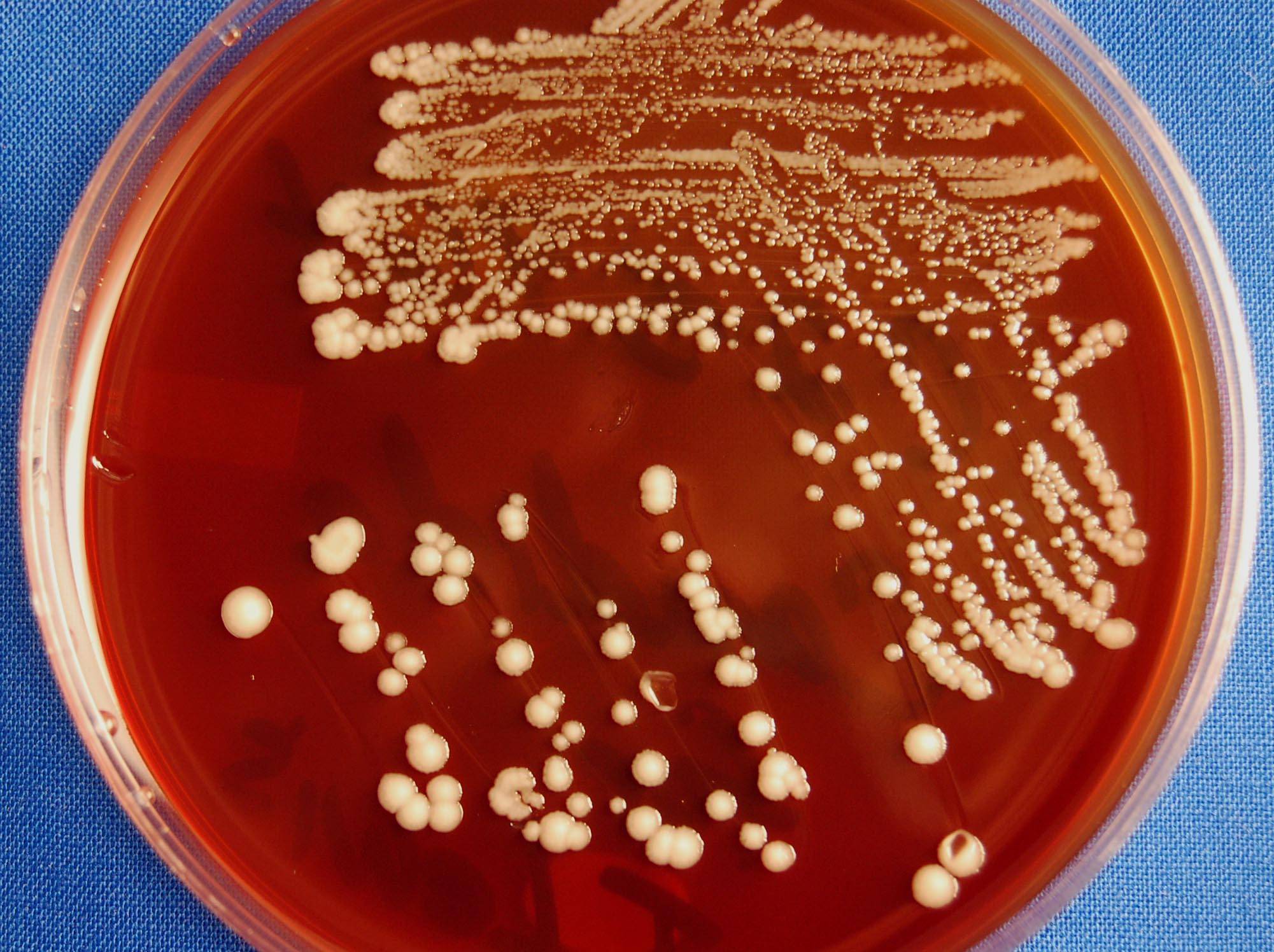
The drug-resistant fungus Candida auris (C. auris) was only discovered some 15 years ago but is already one of the world’s most feared hospital microbes.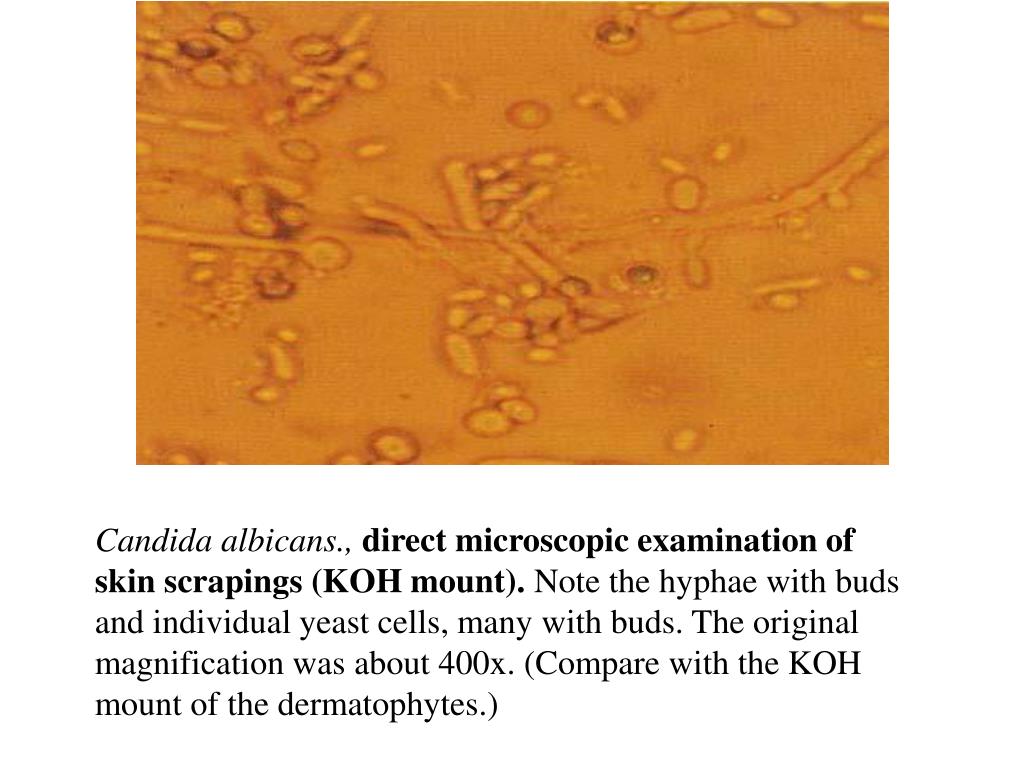
If it gets inside the body, the yeast-type fungus can affect the bloodstream, the nervous system and several internal organs. The World Health Organization (WHO) estimates that its mortality rate ranges from 30% to 53% of patients affected by an invasive infection.
What is more worrisome is that the fungus has proven to be resistant to the most common types of antifungal drugs. Some strains are resistant to all of the medicines we have, says BBC’s health correspondent James Gallagher.
According to the US Centers for Disease Control and Prevention (CDC), there have been outbreaks in more than 30 countries. A 2020 review from case reports from those nations found almost 4,750 cases globally between 2009 and 2019.
- Deadly fungal infection spreading in US
- Fungal ‘threat’ to human health
The CDC said new data showed the fungus has “spread at an alarming rate in US healthcare facilities” in 2020 and 2021. Clinical cases in the country trebled – from 476 in 2019 to 1,471 in 2021.
Also, a 2019 study by an international team of researchers suggested that rising temperatures linked to climate change may have played a role in the rising number of Candida auris infections.
Here is everything you need to know about this deadly superbug.
What is Candida auris?
Candida auris (C. auris) is a yeast, a family of fungus which contains species pretty helpful to humans in activities such as bread-making and beer-brewing, but which also features species that cause infections in humans.
Image source, Getty Images
Image caption,
Some yeast fungi species have been pretty useful to humans
One example is the very common Candida albicans, which causes thrush but also may trigger more severe infections.
C. auris was first discovered in the ear canal of a patient at the Tokyo Metropolitan Geriatric Hospital in 2009, which inspired its name (auris is Latin for ear).
Most of the time, Candida yeasts live on our skin without causing problems, but they can cause infections if we are unwell or they get into the wrong place, like the bloodstream or the lungs.
What sort of illness does it cause?
C. auris most frequently causes bloodstream infections, but it can also affect the respiratory system, the central nervous system and internal organs, as well as the skin.
These infections are usually quite serious.
The fungus is often resistant to the usual drugs, which makes infections difficult to treat.
“The biggest problem with this fungus is its resistance to the drugs we have,” said Dr Tina Joshi, associate professor in Molecular Biology at the University of Plymouth, in the UK.
“But another issue is that identifying a C. auris infection is quite difficult and it can easily be mistaken for other fungi, leading to the wrong treatment.”
Image source, Getty Images
Image caption,
C. auris is often resistant to the most common anti-fugal drugs, which makes infections difficult to treat
This means that the patient might be ill for longer, or get worse before accessing the appropriate treatment.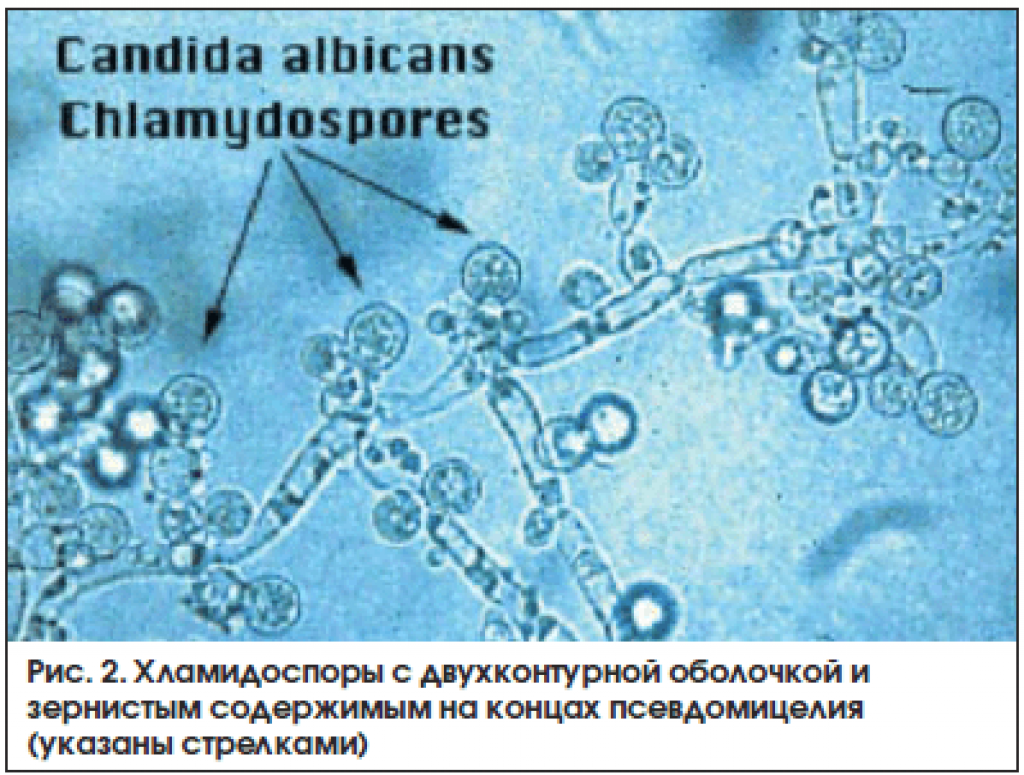
How does it spread?
Transmission is mainly through contaminated surfaces in hospitals. It sticks to intravenous lines and blood pressure cuffs. It’s really hard to clean off, according to Dr Neil Stone, leading fungal expert at the Hospital for Tropical Diseases, University College London.
The solution is often to close off entire wards.
“It is the most worrying fungi and we ignore it at our peril,” Dr Stone said.
“It could shut down entire healthcare systems.”
In a statement issued on 20 March, the CDC said new data showed that the fungus has “spread at an alarming rate” in the US.
Should I be worried about getting an infection?
It is unlikely that you will pick up a C. auris infection going about your daily life.
However, the risk is higher if you are in a hospital for a long time or if you are in a nursing home, and patients who are in intensive care are much more likely to get a C. auris infection, according to the CDC.
auris infection, according to the CDC.
Image source, Getty Images
Image caption,
The fungus sticks to all kinds of surfaces in hospitals, including intravenous lines
The risk of picking up an infection is also higher if you have been on antibiotics a lot, because the drugs also destroy good bacteria that can stop C. auris getting in.
Why is C. auris resistant to the usual drugs?
Resistance to the common antifungal drugs, like fluconazole, has been seen in the majority of C. auris strains.
This means that these drugs do not work on C. auris. Because of this, less common antifungal drugs have been used to treat infections, but C. auris has now developed resistance to these, too.
DNA evidence shows that the antifungal resistance genes in C. auris are very similar to those found in the very common C. albicans.
This suggests that the resistance genes may have passed from one species to the other.
How can climate change be responsible for the high numbers of infections?
A 2019 study, published by the journal mBio from the American Society for Microbiology, suggested that the reason C. auris infections have become so common may be because this species has been forced to live at higher temperatures because of climate change.
auris infections have become so common may be because this species has been forced to live at higher temperatures because of climate change.
Most fungi prefer the cooler temperatures found in soil. But, as global temperatures have risen, C. auris has been forced to adapt to higher temperatures.
Image source, Getty Images
Image caption,
More infection prevention efforts are needed in the battle against C. auris, experts say
This may have made it easier for the fungus to thrive in the human body, which is warm at 36C to 37C.
What can be done to control the number of infections?
A better understanding of who is most at risk of contracting a C. auris infection is the first step towards reducing the number of infections.
“We are behind the curve in what concerns the study of fungi,” Dr Joshi said.
“I am not surprised at all we are now having to catch-up with it.”
Healthcare professionals need to know that people who spend long periods of time in hospitals or nursing homes or those who have a weakened immune system are at higher risk.
Not all hospitals identify C. auris in the same way. They are sometimes mistaken for other fungal infections, like thrush, and the wrong treatment is given.
Image source, Getty Images
Image caption,
C. auris is related to the more commonly found fungus Candida albicans
Improving diagnosis will help to identify patients with C. auris earlier, which will mean that the right treatment is given – preventing the spread of infection to other patients.
But above all, infection prevention efforts need to be improved, said Dr Joshi.
“The main measure is infection prevention and control, because we have already seen how difficult it is to tackle what it does to patients.”
“Hospitals need to be on top of disinfecting and cleaning.”
Is this the only nasty fungus around?
Not quite. In its first-ever list of fungal “priority pathogens”, published last October, the WHO named no less than 19 fungi that represent a great threat to public health.
C. auris was one of four fungi to appear in the “critical priority” group, being described by the WHO as “intrinsically resistant to most available antifungal medicines”.
This article has been adapted from material produced by Lena Ciric and James Gallagher.
- Infection
- United States
Candida albicans: photo of candida albicans, tests for Candida Albikans, treatment for Candida albicans
What is Candida Albicans and what is the danger to humans?
Candida albicans is a type of yeast-like fungus from the genus Candida that causes candidiasis: a dangerous human disease that can affect the mucous membranes of the mouth and genitals, skin, and in its most severe forms, blood and internal organs.
Despite the fact that Candida fungi are considered opportunistic, an increasing number of experts declare the unequivocal danger of this microorganism associated with its aggressiveness and ability to explode in the human body. Almost any external or internal factor (for example, stress, hypothermia, pregnancy) can provoke the active development of candida albicans in women and men and lead to pathological conditions.
Almost any external or internal factor (for example, stress, hypothermia, pregnancy) can provoke the active development of candida albicans in women and men and lead to pathological conditions.
Features of the structure and development
The yeast fungus Candida albicans has an elongated shape and is able to form pseudomycelium, as well as false spores – cells with a compacted membrane protected from aggressive environmental influences. Spores contribute to the spread of candida, as they are able to remain viable for a long time. When it enters the human body, the spores are activated and candida white begins to actively develop and multiply.
For a long time, the presence of candida albicans in the intestines was considered the norm: more than 80% of the world’s population are carriers of this fungus. The yeast-like microorganism is involved in metabolic processes, helps break down sugars in the small intestine, and up to a certain point manifests itself as a symbiont organism. But any violation of homeostasis causes aggressive growth of the microorganism and leads to the development of thrush (candidiasis infection), and with reduced immunity, the development of visceral candidiasis is also possible – a systemic disease that leads to inhibition of the functions of various organs.
But any violation of homeostasis causes aggressive growth of the microorganism and leads to the development of thrush (candidiasis infection), and with reduced immunity, the development of visceral candidiasis is also possible – a systemic disease that leads to inhibition of the functions of various organs.
Favorable conditions for the development of the fungus is a slightly alkaline and neutral environment, a change in the direction of acidity causes the death of the microorganism.
Factors provoking the development of candida
If a person is an asymptomatic carrier of candida, then the mechanism of its “activation”, leading to the development of candidiasis, can be triggered by numerous factors:
- Reduced immunity due to stress, imbalance of micro and macro elements, change of time zone, and so on.
Any disturbance of homeostasis associated with a temporary decrease in immunity (for example, seasonal beriberi) can trigger the development of candida. Therefore, many people are faced with candidiasis after suffering ARVI, influenza and other colds.
Therefore, many people are faced with candidiasis after suffering ARVI, influenza and other colds. - Taking antibiotics or undergoing other major therapies.
Antibiotics not only inhibit the growth of pathogenic microorganisms, but also destroy the beneficial intestinal microflora, which gives the green light to candida, which begin to grow, causing unpleasant symptoms of Candida albicans infection. - Hormonal imbalance.
Changes in the hormonal background can be caused both by natural causes (for example, for a woman, this is the onset of pregnancy, which often provokes the development of candida albicans), and by taking certain drugs (these include oral contraceptives).
It is noteworthy that sometimes almost any trifle can become the cause of the development of candida albicans: for example, choosing the wrong toothpaste or constantly wearing dentures can lead to oral candidiasis. If we consider how candida albicans is transmitted, then the main mechanism of transmission remains oral and sexual contact.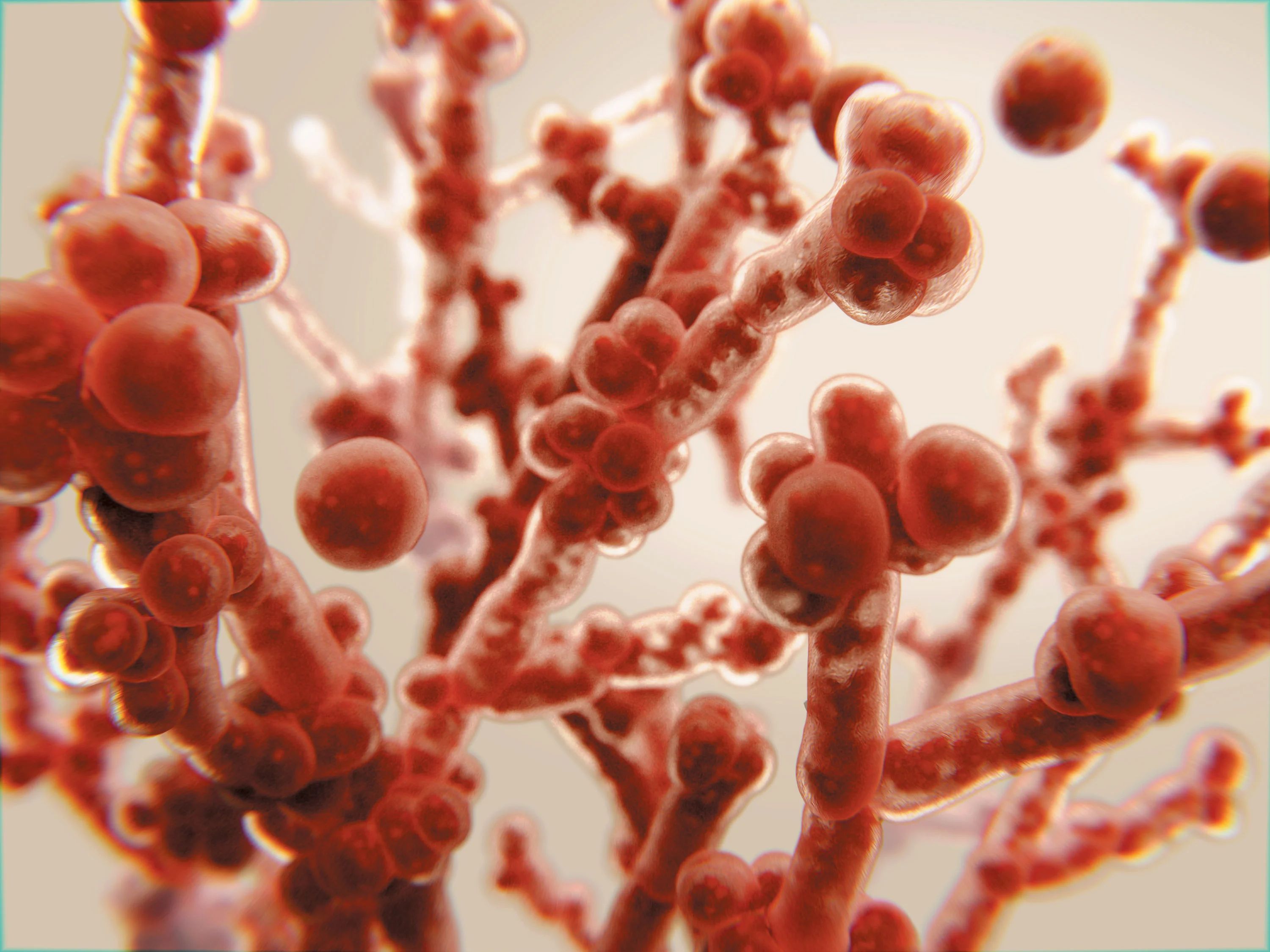
Candida symptoms
Candida albicans is found in large numbers in the human body, and can parasitize on the mucous membranes of various organs. Most often, the fungus is found in the microflora of the intestines and oral cavity, but is also present on the genitals, can be found in the blood and in various organs of the body. Depending on this, Candida infection can manifest itself with various symptoms.
One of the most famous forms of infection is urogenital. Usually this disease is called thrush (candida in women and men). The symptoms of invasion are as follows: irritation and itching of the genital organs, pain during intercourse and urination, the appearance of characteristic whitish discharge, resembling curd mass in consistency.
Another fairly common form of candida infection is oral. In terms of symptoms, it resembles urogenital (tissue irritation, redness, characteristic whitish coating), but is localized not on the genitals, but in the oral cavity.
An intestinal infection of candida albicans also occurs. In this case, the patient experiences periodic pain in the abdomen, suffers from increased gas formation and stool disorders (it becomes irregular, frothy). There is a serious imbalance of micro and macro elements. Gradually, the symptoms worsen, which is also associated with the accumulation of fungal toxins in the body, which are absorbed in the intestines and affect all organ systems. A person becomes irritable, experiences weakness and fatigue, suffers from headaches.
Candida albicans on the skin and other forms of the disease
Candida Albicans can parasitize not only on the mucous membranes, but also on the skin and nails. The manifestations of the disease are as follows: irritation appears on the dermis, the upper scales of the epithelium peel off, and sensitivity increases. The skin becomes wet, emits a characteristic unpleasant odor.
But the most dangerous form of manifestation of fungal infection remains visceral or systemic candidiasis. In this case, candida spreads to the mucous membranes of all organs and affects the cardiovascular, respiratory, and digestive systems. The fungus can even be found on the meninges. This causes septic changes and can be fatal.
In this case, candida spreads to the mucous membranes of all organs and affects the cardiovascular, respiratory, and digestive systems. The fungus can even be found on the meninges. This causes septic changes and can be fatal.
Test for candida albicans
Many methods have been developed for laboratory confirmation of the presence of candida in the human body. With skin lesions, as a rule, they take a scraping, in which a fungus is found. Also an effective way to establish invasion is PCR, which determines the DNA of Candida albicans. In some cases, the patient is prescribed a blood test, in which specific antibodies are detected.
Candida albicans
It would be a mistake to assume that for the treatment of candida albicans in men or women, it is enough to remove the external manifestations of invasion. In this case, the disease will return after some time. To prevent this from happening, a systematic approach to the patient’s recovery is needed. Treatment for candida albicans in women should also be aimed at eliminating all the consequences of invasion, restoring immunity, balancing micro and macro elements, and hormonal levels.
Treatment for candida albicans in women should also be aimed at eliminating all the consequences of invasion, restoring immunity, balancing micro and macro elements, and hormonal levels.
NPK “Optisalt” has developed drugs that can effectively remove the fungus and its spores from the human body. The Optisalt complex can also be used to prevent invasions, if candida was detected in one of the sexual partners or family members.
During an evolutionary experiment, a pathogenic fungus turned into a beneficial symbiont
Evolutionary experiments conducted by Singaporean biologists showed that a potentially pathogenic fungus Candida albicans in just a few weeks of life in the intestines of a mouse can turn into a beneficial symbiont, protecting the host from fungal and bacterial infections through the activation of innate immune systems. This evolutionary transformation occurs on the condition that the bacteria in the mouse intestines have been previously destroyed by antibiotics. In the absence of competition from bacteria, selection favors mutations that deprive the fungus of its ability to form hyphae and, along the way, give it properties that are potentially beneficial to the host. In the presence of normal bacterial microflora in the intestines, the same mutations make the fungus uncompetitive. Therefore, if you do not feed the mouse with antibiotics, then the fungus, depending on the age of the mouse, either does not survive in its intestines at all, or survives, but does not evolve into a useful symbiont.
In the absence of competition from bacteria, selection favors mutations that deprive the fungus of its ability to form hyphae and, along the way, give it properties that are potentially beneficial to the host. In the presence of normal bacterial microflora in the intestines, the same mutations make the fungus uncompetitive. Therefore, if you do not feed the mouse with antibiotics, then the fungus, depending on the age of the mouse, either does not survive in its intestines at all, or survives, but does not evolve into a useful symbiont.
Relationships between animals and microorganisms living in their intestines vary from antagonistic to mutualistic (mutually beneficial). Understanding the reasons that determine whether our microbes will be at enmity with us or be friends is important for the development of medicine. However, little is known about the factors that direct the evolution of gut microbes towards mutually beneficial cooperation with the host.
On the one hand, biologists have already achieved some success in the artificial construction of mutualistic systems (see the news: Mutually beneficial symbiosis of a fungus and algae can form instantly, “Elements”, 07/07/2014, E. coli was taught to integrate into a yeast cell and work as a mitochondrion, ” Elements”, 10/30/2018), and in a number of evolutionary experiments it was possible to observe the development of cooperation between microbes (The evolution of species in a community is not the same as in a monoculture, “Elements”, 19.05.2012). On the other hand, there have been no convenient experimental systems for studying how useless or harmful gut microbes become beneficial symbionts in the course of evolution.
coli was taught to integrate into a yeast cell and work as a mitochondrion, ” Elements”, 10/30/2018), and in a number of evolutionary experiments it was possible to observe the development of cooperation between microbes (The evolution of species in a community is not the same as in a monoculture, “Elements”, 19.05.2012). On the other hand, there have been no convenient experimental systems for studying how useless or harmful gut microbes become beneficial symbionts in the course of evolution.
Fungus Candida albicans is often found in humans in the digestive tract and genital tract. It usually behaves like a peaceful commensal, but occasionally causes serious illness (see Candidiasis). Studying the relationship of C. albicans with the host is difficult because familiar laboratory animals, such as mice, are not the natural hosts of this fungus. In the intestine of an adult mouse C. albicans does not survive, apparently unable to compete with common gut microbes. However, scientists still found two ways to settle C. albicans in the mouse intestine. First, you can feed the mouse with antibiotics to kill the bacterial microflora. After that, C. albicans successfully colonizes the mouse’s digestive tract. Secondly, it is possible to infect a young mouse with a fungus, in which the normal microflora has not yet formed. In this case, you can do without antibiotics. In addition, you can enter cells C. albicans into the blood, but this ends badly for the mouse: the fungus grows in the kidneys and other organs, and the animal dies.
However, scientists still found two ways to settle C. albicans in the mouse intestine. First, you can feed the mouse with antibiotics to kill the bacterial microflora. After that, C. albicans successfully colonizes the mouse’s digestive tract. Secondly, it is possible to infect a young mouse with a fungus, in which the normal microflora has not yet formed. In this case, you can do without antibiotics. In addition, you can enter cells C. albicans into the blood, but this ends badly for the mouse: the fungus grows in the kidneys and other organs, and the animal dies.
Microbiologists and immunologists from Singapore decided to find out how the fungus C. albicans adapts to its new habitat – the intestines of mice devoid of bacterial microflora.
To begin with, mice receiving regular antibiotics were fed a serving of C. albicans. Infection was successful: the fungus took root in the intestines of experimental animals. After that, every week the fungus was transplanted into the next host – another mouse sitting on antibiotics – feeding it the feces of the previous one.
The growth of the adaptability of the fungus to new conditions was monitored by assessing the rate of its reproduction in the intestines of bacteria-free mice compared to the original strain. Already after 10 weeks (that is, after 10 transplants from one mouse to another), the fitness of experimental strains C. albicans increased by 10-15%. Thus, the fungi successfully adapted to the new environment. Now it was necessary to find out how they succeeded and why.
First, the scientists tested whether the interaction with the adaptive (acquired) immunity system of the host plays any role in the adaptation of the fungus to the mouse intestine. To do this, the experiment was repeated on mice that not only sat on antibiotics, but were also deprived of the ability to develop acquired immunity. These mice don’t have gene Rag1 required for the formation of functional B- and T-lymphocytes. The result was reproduced: the fitness of the fungus increased in 10 weeks approximately in the same way as in the case of ordinary bacterial-free mice. This means that successful adaptation of the fungus to life in the mouse intestine does not require interaction with the adaptive immunity of the host.
This means that successful adaptation of the fungus to life in the mouse intestine does not require interaction with the adaptive immunity of the host.
Will the bacteria normally found in the mouse intestine interfere with the successful adaptation of the fungus? The authors failed to infect adult mice with normal intestinal microflora (not taking antibiotics) with the fungus, as no one had succeeded before. Apparently, the existing bacterial microflora does not allow the fungus to multiply in the mouse intestine. Then the researchers switched to experiments with two-week-old mice, in whose intestines C. albicans survives even without antibiotics.
It turned out that if mice infected with a fungus are not given antibiotics, the fungus in their intestines, although it survives, cannot adapt to the new environment as well as in the case of antibiotics. After 5–10 weeks of life in antibiotic-fed mice, the fitness of C. albicans to the new environment increased almost as much as after 10 weeks of life in adult bacterial-free mice. However, after the same 5–10 weeks of life in mice not treated with antibiotics, the fitness of the fungus remained at its original level.
However, after the same 5–10 weeks of life in mice not treated with antibiotics, the fitness of the fungus remained at its original level.
These and other experiments have shown that in the presence of normal murine intestinal bacteria, the fungus fails to acquire any properties that increase its competitiveness in the intestines of germ-free mice.
What are these properties? It turned out that all strains C. albicans , whose adaptability to life in the mouse intestine increased in the course of the experiments (both in adult mice without bacteria, and in mice, and in mice without adaptive immunity) lost the ability to form multicellular filaments – hyphae. These strains can only exist in the yeast form, that is, as individual cells (Fig. 1). Apparently, the ability to form hyphae helps the fungus survive in the presence of intestinal bacteria, but slows down reproduction in the gut of a microbial mouse.
To understand the genetic basis of the changes that occurred, the authors sequenced the genomes of 28 C. albicans strains that successfully adapted to life in the intestines of germ-free mice and compared them with the genome of the original strain.
albicans strains that successfully adapted to life in the intestines of germ-free mice and compared them with the genome of the original strain.
It turned out that all adapted strains had significant (nonsynonymous, changing the encoded protein) mutations in at least one of 34 protein-coding genes, the functions of which are associated with hyphae formation, cell wall construction, and transcription regulation (Fig. 2).
Most often (in 22 out of 28 strains), mutations that disable the FLO8 gene were fixed. This gene encodes a transcription factor that regulates hyphalation. All mutations fixed in it make the encoded protein non-functional (through the appearance of a premature stop codon or a reading frame shift). Additional experiments confirmed that fungi with the inactivated FLO8 gene, firstly, do not form hyphae, and secondly, have an increased competitiveness in the intestines of bacteria-free mice.
Thus, the course of adaptation of C. albicans to life in the intestines of bacteria-free mice turned out to be predictable and reproducible: the ability to form hyphae is always lost, and most often this occurs due to mutations that disable the FLO8 gene.
albicans to life in the intestines of bacteria-free mice turned out to be predictable and reproducible: the ability to form hyphae is always lost, and most often this occurs due to mutations that disable the FLO8 gene.
Both hyphae formation and FLO8 have previously been shown to influence the virulence of C. albicans , determining the ability of the fungus to cause systemic infection when ingested into the blood (F. Cao et al., 2006. The Flo8 Transcription Factor Is Essential for Hyphal Development and Virulence in Candida albicans ). Therefore, the authors suggested that strains adapted to life in the mouse intestine could become less harmful.
Co-cultivation experiments of C. albicans with mammalian cells (mouse macrophages and human colonic epithelial cells) confirmed that strains of C. albicans adapted to life in the intestines of bacteria-free mice do not cut cells like the original strain. The introduction of the original strain into the blood leads to the growth of fungal hyphae in the kidneys after two days, and after 3–4 days all mice die. Injection of the same number of cells C. albicans adapted to bacteria-free mouse intestine, mice survive safely. At the same time, the fungus is found in tissues only in the yeast form. The strains that evolved in the intestines of mice that did not receive antibiotics not only did not forget how to form hyphae, but also fully retained their lethality when they entered the bloodstream.
Injection of the same number of cells C. albicans adapted to bacteria-free mouse intestine, mice survive safely. At the same time, the fungus is found in tissues only in the yeast form. The strains that evolved in the intestines of mice that did not receive antibiotics not only did not forget how to form hyphae, but also fully retained their lethality when they entered the bloodstream.
Thus, in the course of adaptation to life in the intestines of bacteria-free mice, the fungus C. albicans actually became less dangerous for the host.
The authors did not stop there and decided to check whether it also became useful. They proceeded from the fact that commensal fungi living in the intestines, in principle, can protect the host from infections (T. T. Jiang et al., 2017. Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria). Experiments have shown that strains of C. albicans adapted to the intestines of bacteria-free mice do indeed have protective properties that strains that retain the ability to form hyphae do not have. These protective properties are manifested when the fungi simply live in the intestines of mice, and even more so when they are injected into the bloodstream as a vaccine. This protection is non-specific and applies to a wide range of pathogens (including “wild” strains C. albicans , other pathogenic fungi, gram-positive and gram-negative bacteria). Mice with the Rag1 gene knockout, which are unable to develop adaptive immunity, are also protected. This means that the protective effect is not associated with adaptive immunity (as with conventional vaccination).
These protective properties are manifested when the fungi simply live in the intestines of mice, and even more so when they are injected into the bloodstream as a vaccine. This protection is non-specific and applies to a wide range of pathogens (including “wild” strains C. albicans , other pathogenic fungi, gram-positive and gram-negative bacteria). Mice with the Rag1 gene knockout, which are unable to develop adaptive immunity, are also protected. This means that the protective effect is not associated with adaptive immunity (as with conventional vaccination).
Apparently, C. albicans strains , which have adapted to life in the mouse intestine freed from bacteria, contribute to the long-term activation of innate immune systems, which, as it has become clear in recent years, also have a kind of immunological memory and can be “trained” better protect the body from certain infections (M. G. Netea et al., 2016. Trained immunity: A program of innate immune memory in health and disease).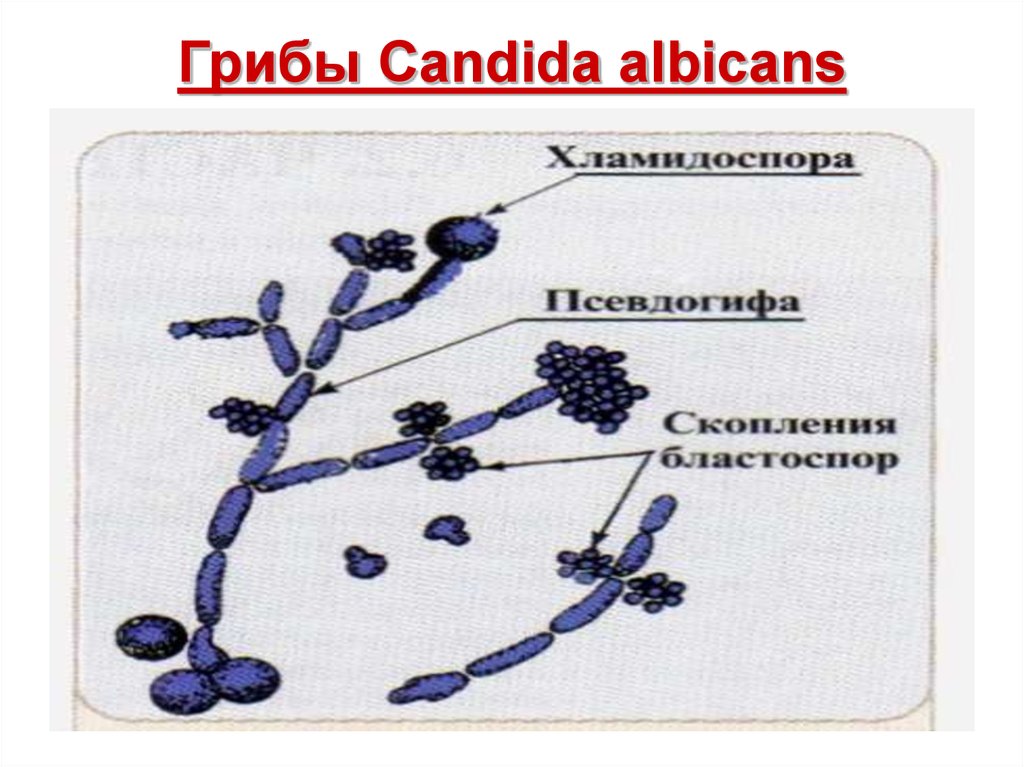 Ability of test strains C. albicans “train” innate immunity is due not only to the absence of hyphal formation and not only to the failure of the FLO8 gene, but also to some additional mutations that these strains have. This follows from the fact that the protective properties of many of them are more pronounced than in the original strain with an artificially disabled hyphae formation system (it was disabled by disabling the FLO8 or EFG1 gene).
Ability of test strains C. albicans “train” innate immunity is due not only to the absence of hyphal formation and not only to the failure of the FLO8 gene, but also to some additional mutations that these strains have. This follows from the fact that the protective properties of many of them are more pronounced than in the original strain with an artificially disabled hyphae formation system (it was disabled by disabling the FLO8 or EFG1 gene).
The authors admit that on the basis of the strains they have bred, it will be possible in the future to develop a new type of vaccine that will protect even people with impaired adaptive immunity (AIDS patients, people after transplantation, etc.)
Thus, the study showed that a health-threatening fungus can quickly evolve into a potentially beneficial intestinal symbiont, unless it is prevented from doing so by complex relationships with other inhabitants of the intestine. It seems that the ability to form hyphae is necessary for the fungus C.

 Therefore, many people are faced with candidiasis after suffering ARVI, influenza and other colds.
Therefore, many people are faced with candidiasis after suffering ARVI, influenza and other colds.