How many antibiotics. The World is Running Out of Antibiotics: A Comprehensive Analysis of Bacterial Infections, Side Effects, Resistance, Types, and Interactions
How many antibiotics are available to treat bacterial infections? Discover the growing threat of antimicrobial resistance, the types of antibiotics, their side effects, and the challenges in developing new treatments.
The Alarming Reality: A World Running Out of Antibiotics
A recent report by the World Health Organization (WHO) has confirmed a disturbing reality – the world is running out of effective antibiotics. The report, titled “Antibacterial agents in clinical development – an analysis of the antibacterial clinical development pipeline, including tuberculosis,” paints a bleak picture of the current state of antibiotic development and the growing threat of antimicrobial resistance.
The Urgent Need for New Antibiotics
According to the WHO, there is a serious lack of new antibiotics under development to combat the increasing threat of antibiotic-resistant infections. Most of the drugs currently in the clinical pipeline are modifications of existing classes of antibiotics, which provide only short-term solutions. The report found very few potential treatment options for those antibiotic-resistant infections identified by the WHO as posing the greatest threat to global health, including drug-resistant tuberculosis (TB).

The Global Health Emergency
Dr. Tedros Adhanom Ghebreyesus, Director-General of the WHO, has called antimicrobial resistance a “global health emergency that will seriously jeopardize progress in modern medicine.” The lack of investment in research and development for antibiotic-resistant infections, including TB, is a major concern, as it could force the world to revert to a time when people feared common infections and risked their lives from minor surgery.
Prioritizing Antibiotic-Resistant Pathogens
The WHO has identified 12 classes of priority pathogens – some of them causing common infections such as pneumonia or urinary tract infections – that are increasingly resistant to existing antibiotics and urgently in need of new treatments. These include multidrug-resistant tuberculosis and gram-negative pathogens, such as Acinetobacter and Enterobacteriaceae (including Klebsiella and E.coli), which can cause severe and often deadly infections.
Innovative Treatments: A Glimmer of Hope?
The report identifies 51 new antibiotics and biologicals in clinical development to treat priority antibiotic-resistant pathogens, as well as tuberculosis and Clostridium difficile infections. However, only 8 of these candidate medicines are considered by the WHO as innovative treatments that will add value to the current antibiotic treatment arsenal. The lack of truly innovative antibiotics is a significant concern.

Combating Antimicrobial Resistance: Ongoing Challenges
The report highlights the urgent need for more investment in research and development for antibiotic-resistant infections, including TB. Without significant progress in this area, the world may be forced to confront a future where common infections and minor surgeries pose serious threats to human health and wellbeing.
What are the different types of antibiotics?
Antibiotics can be classified into several categories based on their mechanism of action, spectrum of activity, and chemical structure. The main types of antibiotics include:
- Penicillins (e.g., amoxicillin, penicillin G)
- Cephalosporins (e.g., cefuroxime, ceftriaxone)
- Macrolides (e.g., erythromycin, azithromycin)
- Fluoroquinolones (e.g., ciprofloxacin, levofloxacin)
- Tetracyclines (e.g., doxycycline, minocycline)
- Aminoglycosides (e.g., gentamicin, amikacin)
- Sulfonamides (e.g., sulfamethoxazole, trimethoprim-sulfamethoxazole)
What are the common side effects of antibiotics?
Antibiotics can cause a range of side effects, including:
- Gastrointestinal issues (e.g., nausea, vomiting, diarrhea)
- Allergic reactions (e.g., rash, hives, anaphylaxis)
- Disruption of the gut microbiome, leading to Clostridium difficile infection
- Kidney and liver toxicity (with certain antibiotics)
- Antibiotic-associated tendinitis or tendon rupture (with fluoroquinolones)
- Neurological effects (e.g., headaches, dizziness, seizures)
/resolutions/res-l1920x10000/Somministrazione_antibiotica_resistenza_intravenosa_UniSR-(3).jpg)
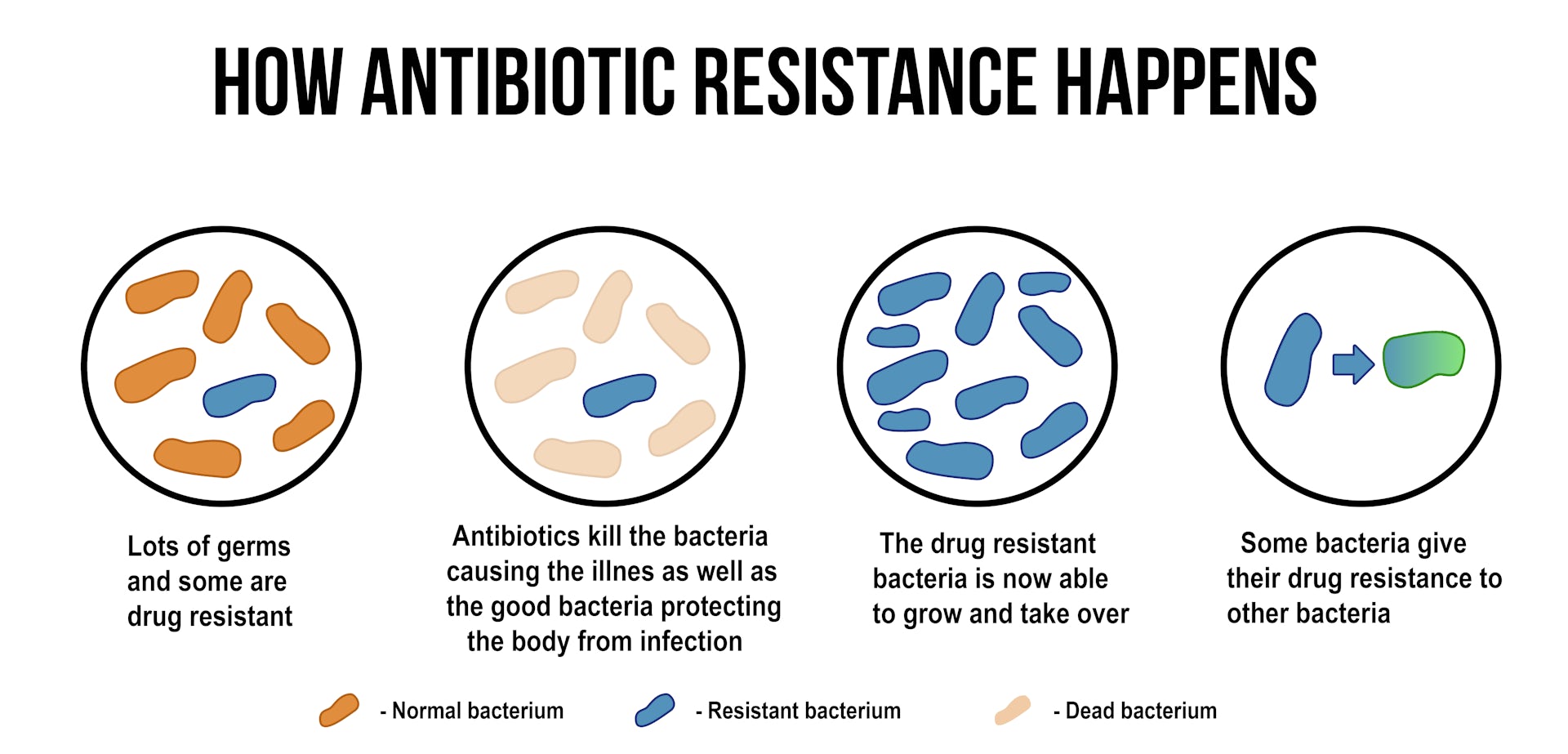
How does antibiotic resistance develop?
Antibiotic resistance occurs when bacteria evolve mechanisms to survive and thrive in the presence of antibiotics. This can happen through several mechanisms, such as:
- Producing enzymes that inactivate or modify the antibiotic
- Altering the antibiotic’s target site within the bacterial cell
- Reducing the uptake of the antibiotic or increasing its efflux from the cell
- Acquiring resistance genes from other bacteria through horizontal gene transfer
The overuse and misuse of antibiotics, as well as poor infection control practices, have contributed to the rise of antibiotic-resistant bacteria, making infections increasingly difficult to treat.
What is the impact of antibiotic resistance on global health?
Antibiotic resistance poses a significant threat to global health. When common infections become resistant to standard antibiotic treatments, it can lead to:
- Increased morbidity and mortality, as infections become more difficult to treat
- Prolonged hospital stays and higher healthcare costs
- The potential return to a pre-antibiotic era, where minor injuries and infections could again become life-threatening
- Reduced effectiveness of medical procedures that rely on effective antibiotic prophylaxis, such as surgery, cancer treatment, and organ transplantation
Addressing the challenge of antibiotic resistance requires a multi-faceted approach, including improved antimicrobial stewardship, infection prevention and control, and accelerated research and development of new antibiotics and alternative therapies.

How can we address the lack of new antibiotics in development?
To address the lack of new antibiotics in development, several strategies can be employed:
- Increased funding and investment in antibiotic research and development, particularly for innovative treatments targeting the priority pathogens identified by the WHO
- Incentives and policies to encourage pharmaceutical companies to invest in antibiotic development, such as tax credits, market entry rewards, and extended patent protection
- Improved collaboration and coordination between the public and private sectors to streamline the antibiotic development pipeline
- Strengthening of regulatory frameworks to facilitate the approval of new antibiotics while maintaining high standards of safety and efficacy
- Promotion of antimicrobial stewardship programs to ensure the appropriate use of antibiotics and minimize the development of resistance
Addressing the antibiotic crisis will require a concerted, global effort to spur innovation and ensure the availability of effective treatments for generations to come.

What is the role of the World Health Organization in tackling antibiotic resistance?
The World Health Organization (WHO) plays a crucial role in addressing the challenge of antibiotic resistance:
- Identifying and prioritizing the most critical antibiotic-resistant pathogens that require new treatments, as outlined in the recent report
- Monitoring and reporting on the global state of antibiotic resistance and the antibiotic development pipeline
- Providing guidance and recommendations on antimicrobial stewardship, infection prevention and control, and the development of new antibiotics and alternative therapies
- Advocating for increased investment and international collaboration to spur innovation in antibiotic research and development
- Promoting global awareness and action on the threat of antimicrobial resistance through initiatives such as World Antibiotic Awareness Week
The WHO’s leadership and coordinated efforts are essential in mobilizing a global response to the antibiotic crisis and ensuring the availability of effective treatments for the future.
How can individuals contribute to addressing antibiotic resistance?
Individuals can play a role in combating antibiotic resistance by:
- Adhering to healthcare providers’ instructions when taking antibiotics, including completing the full course of treatment as prescribed
- Avoiding the misuse or overuse of antibiotics, such as demanding them for viral infections or using leftover antibiotics
- Practicing good hygiene, such as handwashing, to prevent the spread of infections and reduce the need for antibiotics
- Advocating for and supporting policies and initiatives that promote antibiotic stewardship and the development of new antibiotics
- Educating themselves and others about the importance of preserving the effectiveness of antibiotics and the dangers of antibiotic resistance
By taking these actions, individuals can contribute to the global effort to address the antibiotic crisis and ensure the availability of effective treatments for the future.
The world is running out of antibiotics, WHO report confirms
The world is running out of antibiotics, WHO report confirms
- All topics »
- A
- B
- C
- D
- E
- F
- G
- H
- I
- J
- K
- L
- M
- N
- O
- P
- Q
- R
- S
- T
- U
- V
- W
- X
- Y
- Z
- Resources »
- Fact sheets
- Facts in pictures
- Multimedia
- Publications
- Questions & answers
- Tools and toolkits
- Popular »
- Air pollution
- Coronavirus disease (COVID-19)
- Hepatitis
- Monkeypox
- All countries »
- A
- B
- C
- D
- E
- F
- G
- H
- I
- J
- K
- L
- M
- N
- O
- P
- Q
- R
- S
- T
- U
- V
- W
- X
- Y
- Z
- Regions »
- Africa
- Americas
- South-East Asia
- Europe
- Eastern Mediterranean
- Western Pacific
- WHO in countries »
- Statistics
- Cooperation strategies
- Ukraine emergency
- All news »
- News releases
- Statements
- Campaigns
- Commentaries
- Events
- Feature stories
- Speeches
- Spotlights
- Newsletters
- Photo library
- Media distribution list
- Headlines »
- Focus on »
- Afghanistan crisis
- COVID-19 pandemic
- Northern Ethiopia crisis
- Syria crisis
- Ukraine emergency
- Monkeypox outbreak
- Greater Horn of Africa crisis
- Latest »
- Disease Outbreak News
- Travel advice
- Situation reports
- Weekly Epidemiological Record
- WHO in emergencies »
- Surveillance
- Research
- Funding
- Partners
- Operations
- Independent Oversight and Advisory Committee
- WHO’s Health Emergency Appeal 2023
- Data at WHO »
- Global Health Estimates
- Health SDGs
- Mortality Database
- Data collections
- Dashboards »
- COVID-19 Dashboard
- Triple Billion Dashboard
- Health Inequality Monitor
- Highlights »
- Global Health Observatory
- SCORE
- Insights and visualizations
- Data collection tools
- Reports »
- World Health Statistics 2022
- COVID excess deaths
- DDI IN FOCUS: 2022
- About WHO »
- People
- Teams
- Structure
- Partnerships and collaboration
- Collaborating centres
- Networks, committees and advisory groups
- Transformation
- Our Work »
- General Programme of Work
- WHO Academy
- Activities
- Initiatives
- Funding »
- Investment case
- WHO Foundation
- Accountability »
- Audit
- Programme Budget
- Financial statements
- Programme Budget Portal
- Results Report
- Governance »
- World Health Assembly
- Executive Board
- Election of Director-General
- Governing Bodies website
- Member States Portal
- Home/
- News/
- item/
- The world is running out of antibiotics, WHO report confirms
“,”datePublished”:”2017-09-20T00:00:00. 0000000+00:00″,”image”:”https://cdn.who.int/media/images/default-source/imported/amr-1000-jpg.jpg?sfvrsn=5c2e0073_0″,”publisher”:{“@type”:”Organization”,”name”:”World Health Organization: WHO”,”logo”:{“@type”:”ImageObject”,”url”:”https://www.who.int/Images/SchemaOrg/schemaOrgLogo.jpg”,”width”:250,”height”:60}},”dateModified”:”2017-09-20T00:00:00.0000000+00:00″,”mainEntityOfPage”:”https://www.who.int/news/item/20-09-2017-the-world-is-running-out-of-antibiotics-who-report-confirms”,”@context”:”http://schema.org”,”@type”:”NewsArticle”};
0000000+00:00″,”image”:”https://cdn.who.int/media/images/default-source/imported/amr-1000-jpg.jpg?sfvrsn=5c2e0073_0″,”publisher”:{“@type”:”Organization”,”name”:”World Health Organization: WHO”,”logo”:{“@type”:”ImageObject”,”url”:”https://www.who.int/Images/SchemaOrg/schemaOrgLogo.jpg”,”width”:250,”height”:60}},”dateModified”:”2017-09-20T00:00:00.0000000+00:00″,”mainEntityOfPage”:”https://www.who.int/news/item/20-09-2017-the-world-is-running-out-of-antibiotics-who-report-confirms”,”@context”:”http://schema.org”,”@type”:”NewsArticle”};
A report, Antibacterial agents in clinical development – an analysis of the antibacterial clinical development pipeline, including tuberculosis, launched today by WHO shows a serious lack of new antibiotics under development to combat the growing threat of antimicrobial resistance.
Most of the drugs currently in the clinical pipeline are modifications of existing classes of antibiotics and are only short-term solutions. The report found very few potential treatment options for those antibiotic-resistant infections identified by WHO as posing the greatest threat to health, including drug-resistant tuberculosis which kills around 250 000 people each year.
“Antimicrobial resistance is a global health emergency that will seriously jeopardize progress in modern medicine,” says Dr Tedros Adhanom Ghebreyesus, Director-General of WHO. “There is an urgent need for more investment in research and development for antibiotic-resistant infections including TB, otherwise we will be forced back to a time when people feared common infections and risked their lives from minor surgery.”
In addition to multidrug-resistant tuberculosis, WHO has identified 12 classes of priority pathogens – some of them causing common infections such as pneumonia or urinary tract infections – that are increasingly resistant to existing antibiotics and urgently in need of new treatments.
The report identifies 51 new antibiotics and biologicals in clinical development to treat priority antibiotic-resistant pathogens, as well as tuberculosis and the sometimes deadly diarrhoeal infection Clostridium difficile.
Among all these candidate medicines, however, only 8 are classed by WHO as innovative treatments that will add value to the current antibiotic treatment arsenal.
There is a serious lack of treatment options for multidrug- and extensively drug-resistant M. tuberculosis and gram-negative pathogens, including Acinetobacter and Enterobacteriaceae (such as Klebsiella and E.coli) which can cause severe and often deadly infections that pose a particular threat in hospitals and nursing homes.
There are also very few oral antibiotics in the pipeline, yet these are essential formulations for treating infections outside hospitals or in resource-limited settings.
“Pharmaceutical companies and researchers must urgently focus on new antibiotics against certain types of extremely serious infections that can kill patients in a matter of days because we have no line of defence,” says Dr Suzanne Hill, Director of the Department of Essential Medicines at WHO.
To counter this threat, WHO and the Drugs for Neglected Diseases Initiative (DNDi) set up the Global Antibiotic Research and Development Partnership (known as GARDP). On 4 September 2017, Germany, Luxembourg, the Netherlands, South Africa, Switzerland and the United Kingdom of Great Britain and Northern Ireland and the Wellcome Trust pledged more than €56 million for this work.
On 4 September 2017, Germany, Luxembourg, the Netherlands, South Africa, Switzerland and the United Kingdom of Great Britain and Northern Ireland and the Wellcome Trust pledged more than €56 million for this work.
“Research for tuberculosis is seriously underfunded, with only two new antibiotics for treatment of drug-resistant tuberculosis having reached the market in over 70 years,” says Dr Mario Raviglione, Director of the WHO Global Tuberculosis Programme. “If we are to end tuberculosis, more than US$ 800 million per year is urgently needed to fund research for new antituberculosis medicines”.
New treatments alone, however, will not be sufficient to combat the threat of antimicrobial resistance. WHO works with countries and partners to improve infection prevention and control and to foster appropriate use of existing and future antibiotics. WHO is also developing guidance for the responsible use of antibiotics in the human, animal and agricultural sectors.
For more information, download the following reports:
- Antibacterial agents in clinical development – an analysis of the antibacterial clinical development pipeline, including tuberculosis
- Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis
The clinical pipeline analysis data can be explored in an interactive way through:
- WHO Global Observatory on Health Research and Development
Subscribe to our newsletters →
Where have All the Antibiotics Gone?
The discovery of antibiotics some 60 years ago was anticipated to herald the end of infectious diseases. However, microbial evolution and genetic jugglery have dispelled this notion; the constant increase in the appearance of resistant strains has not been matched by the introduction of new therapeutic agents. On the contrary, the dire need for novel antibiotics has coincided with a reduction in antibiotic discovery programs in the pharmaceutical industry. As a result, the treatment of microbial diseases has reached a point where many infections are essentially untreatable by the antimicrobial agents currently available. At the present time, numerous initiatives are being undertaken by physicians and by governments in an attempt to redress this situation. In addition, alternative approaches to antibiotics for the treatment of infectious diseases are being explored intensively.
However, microbial evolution and genetic jugglery have dispelled this notion; the constant increase in the appearance of resistant strains has not been matched by the introduction of new therapeutic agents. On the contrary, the dire need for novel antibiotics has coincided with a reduction in antibiotic discovery programs in the pharmaceutical industry. As a result, the treatment of microbial diseases has reached a point where many infections are essentially untreatable by the antimicrobial agents currently available. At the present time, numerous initiatives are being undertaken by physicians and by governments in an attempt to redress this situation. In addition, alternative approaches to antibiotics for the treatment of infectious diseases are being explored intensively.
Key Words: Alternative therapies, Antibiotic resistance, Mechanisms
The discovery of penicillin in 1929 and streptomycin in 1943 heralded the age of antibiotics and, coincidentally, the founding of the American pharmaceutical industry. Within a decade after World War II, a number of important antibiotics were discovered and developed for therapeutic use. They became the foundation for the treatment of infectious disease (). This, along with the introduction of better hygiene, led to a dramatic reduction in worldwide morbidity and mortality due to bacterial infections.
Within a decade after World War II, a number of important antibiotics were discovered and developed for therapeutic use. They became the foundation for the treatment of infectious disease (). This, along with the introduction of better hygiene, led to a dramatic reduction in worldwide morbidity and mortality due to bacterial infections.
TABLE 1
Principal antibiotics in use today
| Class | Year discovered |
|---|---|
| Sulfonamides | 1937 |
| Penicillins | 1940 |
| Polymyxin | 1947* |
| Chloramphenicol | 1949 |
| Tetracyclines | 1953 |
| Cephalosporins (four generations) | 1953 |
| Aminoglycosides | 1957 |
| Vancomycin | 1958* |
| Clindamycin | 1966 |
| Rifamycin | 1971 |
| Trimethoprim/sulfamethoxazole | 1973 |
| Carbapenems | 1976 |
| Monobactams | 1982 |
| Linezolid | 1987* |
| Daptomycin | 1987* |
| Synercid | 1992* |
Open in a separate window
*Recently reintroduced
The period from 1950 to 1960 was truly the golden age of antibiotic discovery, as one-half of the drugs commonly used today were discovered in this period.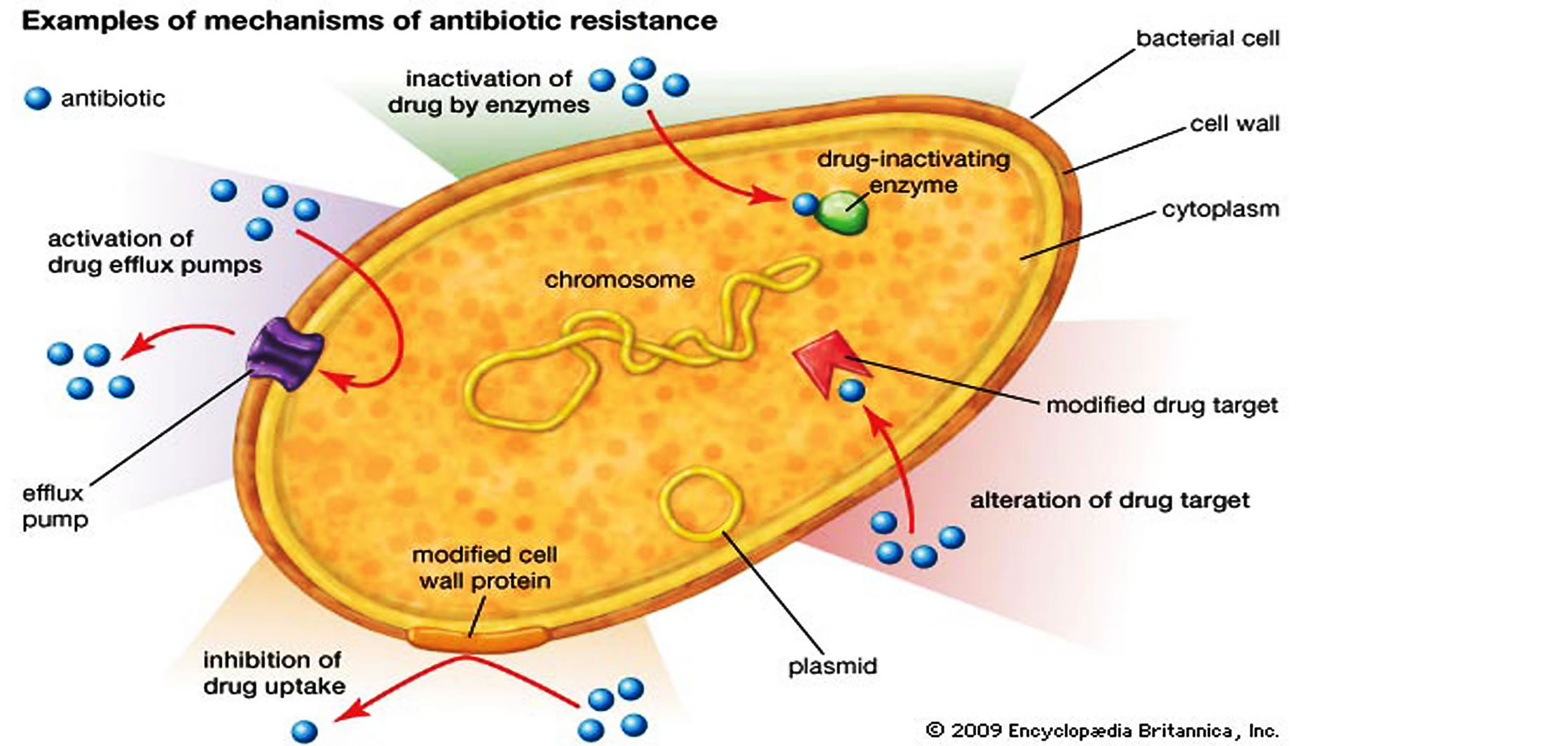 Unfortunately, the increasing use of antibiotics for human and nontherapeutic animal use (growth promotion) led all too soon to the development of resistant bacterial pathogens. Recognizing the correlation between antibiotic use and resistance development, much of subsequent antibiotic research has been devoted to the discovery and design of new compounds effective against the successive generations of resistant pathogens. It is interesting to note that microbial geneticists in the 1950s thought that the development of antibiotic-resistant strains concomitant with antibiotic use would be an unlikely and rare event at most!
Unfortunately, the increasing use of antibiotics for human and nontherapeutic animal use (growth promotion) led all too soon to the development of resistant bacterial pathogens. Recognizing the correlation between antibiotic use and resistance development, much of subsequent antibiotic research has been devoted to the discovery and design of new compounds effective against the successive generations of resistant pathogens. It is interesting to note that microbial geneticists in the 1950s thought that the development of antibiotic-resistant strains concomitant with antibiotic use would be an unlikely and rare event at most!
In the year 2000, antibiotic production in the United States totalled 50 million pounds. Accurate figures are hard to obtain, but assuming this level of production for the past 20 years, it can be estimated that one billion pounds were made during this time. When we consider that the United States is not the principal antibiotic manufacturer (China, India and other countries are heavily involved), the quantity of antibiotics produced and used worldwide may be at least three times greater. The total amount of antibiotics produced since the beginning of the antibiotic era in 1950 is obviously very considerable, and one wonders if it may be significantly more than what is produced naturally in the biosphere, given that antibiotics are made in barely detectable amounts in soil. With respect to distribution, approximately 50% has been devoted to human use, with the remainder applied in animal husbandry, agriculture and aquaculture, etc.
The total amount of antibiotics produced since the beginning of the antibiotic era in 1950 is obviously very considerable, and one wonders if it may be significantly more than what is produced naturally in the biosphere, given that antibiotics are made in barely detectable amounts in soil. With respect to distribution, approximately 50% has been devoted to human use, with the remainder applied in animal husbandry, agriculture and aquaculture, etc.
It is hard to envision the effects of this flood of bioactive molecules on the environment and on microbial ecology. Responding to the selective pressure for survival of the microbial population in the face of this onslaught, bacteria have prevailed and even flourished through a variety of mechanisms of previously unsuspected genetic jugglery, with the concomitant selection of very high levels of antibiotic-resistant organisms in the biosphere.
Transferable resistance was first identified in Japan in the 1950s, although it took a while to decipher the mechanisms involved. The combination of mutation and horizontal gene transfer (HGT) has provided a remarkable collection of biochemical defense mechanisms against antibiotic action in bacterial cells (). The likelihood that additional mechanisms of resistance exist cannot be discounted. Recognition of the fundamental role of HGT in the dissemination of antibiotic resistance in bacteria (by transformation, transduction or conjugation of the encoding genes) has proven to be a finding of great significance. There is convincing evidence that it has been a major factor in prokaryotic evolution, and this has completely changed current concepts of phylogenetic relationships within the prokaryotes: the branches of the ‘trees’ have become interconnected (1)! There are also indications that HGT was critical in the evolution of eukaryotes.
The combination of mutation and horizontal gene transfer (HGT) has provided a remarkable collection of biochemical defense mechanisms against antibiotic action in bacterial cells (). The likelihood that additional mechanisms of resistance exist cannot be discounted. Recognition of the fundamental role of HGT in the dissemination of antibiotic resistance in bacteria (by transformation, transduction or conjugation of the encoding genes) has proven to be a finding of great significance. There is convincing evidence that it has been a major factor in prokaryotic evolution, and this has completely changed current concepts of phylogenetic relationships within the prokaryotes: the branches of the ‘trees’ have become interconnected (1)! There are also indications that HGT was critical in the evolution of eukaryotes.
TABLE 2
Biochemical mechanisms of antibiotic resistance
| Increased efflux | Decreased efflux |
| Enzymatic inactivation | Sequestration |
| Target modification | Target bypass |
| Target repair | Target amplification |
| Biofilm formation | Intracellular localization |
Open in a separate window
It is common to find clusters of resistance genes constructed on transferable vectors by recombination. One well-known structure is the integron, a vehicle by which open reading frames (gene cassettes) can be converted into resistance genes by acquisition and insertion adjacent to strong promoters, ensuring efficient transcription (). The result is that multiple antibiotic resistance is common, perhaps encompassing all useful therapies for certain infections. Multidrug-resistant integrons are common in the Enterobacteriaceae family (2).
One well-known structure is the integron, a vehicle by which open reading frames (gene cassettes) can be converted into resistance genes by acquisition and insertion adjacent to strong promoters, ensuring efficient transcription (). The result is that multiple antibiotic resistance is common, perhaps encompassing all useful therapies for certain infections. Multidrug-resistant integrons are common in the Enterobacteriaceae family (2).
Open in a separate window
The process of resistance gene capture by an integron. The genetic structure of the basic type I integron is shown at the top of the figure. Pant is the promoter that transcribes any gene sequence inserted at the attachment site (attI). The resistance gene cassette (middle) is inserted into the integron structure as a result by recombination between the attC and attI sequences catalyzed by the integrase (intI1 gene). Once integrated, the resistance gene is expressed. Multiple antibiotic resistance occurs by the insertion of additional resistance gene cassettes at the attI site. sul1 encodes resistance to sulfonamide drugs, and gacEΔ is a defective export system. The latter two are found on all type I integrons. This figure was kindly provided by Dr Patrice Courvalin (Institut Pasteur, Paris, personal communication)
Multiple antibiotic resistance occurs by the insertion of additional resistance gene cassettes at the attI site. sul1 encodes resistance to sulfonamide drugs, and gacEΔ is a defective export system. The latter two are found on all type I integrons. This figure was kindly provided by Dr Patrice Courvalin (Institut Pasteur, Paris, personal communication)
Similar types of gene-trapping systems have been detected in Gram-positive pathogens (3). Resistance is often associated with genes for pathogenicity and other survival functions in composite structures known as genomic islands. Thus, the acquisition of antibiotic resistance genes by bacteria can lead to enhanced virulence and vice versa. A supreme example is found in the recent appearance of multidrug-resistant strains of Acinetobacter baumannii, which have become a major nosocomial pathogen; some strains possess a genomic island of 85 genes that encodes resistance to six different antibiotic classes (4). The only effective treatment for multidrug-resistant Acinetobacter species is colistin, a drug rarely used because of its toxicity. In addition to complicating the problems of therapy, the frequent appearance of these gene-trapping systems in genome sequencing studies has overturned accepted concepts of genome evolution.
The only effective treatment for multidrug-resistant Acinetobacter species is colistin, a drug rarely used because of its toxicity. In addition to complicating the problems of therapy, the frequent appearance of these gene-trapping systems in genome sequencing studies has overturned accepted concepts of genome evolution.
Bacteria cannot be considered as independent, isolated colonies on Petri plates. Different environments are components of a huge bacterial network including environmental populations (soil, water), clinical populations (hospital, intensive care unit, community) and commensals of other living species (). The movement of bacterial strains and extensive gene flux takes place between these different populations, such that they can share survival characteristics, typically antibiotic resistance and virulence. An advantageous function from one environment can rapidly make its way throughout the bacterial kingdom. The evidence for reservoirs of potential antibiotic resistance genes in soil is very convincing (5). Biofilms are a good example of an evolutionary mechanism by which mixed populations of prokaryotes acquire a collective advantage in avoiding antibiotics (6). The widespread distribution of antibiotics in hospitals and agricultural environments leads to a situation of constant selective pressure for survival.
Biofilms are a good example of an evolutionary mechanism by which mixed populations of prokaryotes acquire a collective advantage in avoiding antibiotics (6). The widespread distribution of antibiotics in hospitals and agricultural environments leads to a situation of constant selective pressure for survival.
Open in a separate window
The interactive network of pathogens and antibiotic resistance genes
Antibiotic resistance has occurred in our lifetimes. Within two to three years after the introduction of a new antibiotic treatment, resistance usually develops (although there have been a few notable exceptions – penicillin resistance in streptococci, for example). This is nowhere more apparent than in the steady evolution of beta-lactamases (enzymes that detoxify the beta-lactam antibiotics) by point mutation under the selective pressure of successive introductions of new beta-lactamase-resistant penicillins, cephalosporins, carbapenems and monobactams (7). A single nucleotide substitution leading to an amino acid change in such an enzyme necessitates the development of a new antibiotic derivative at a cost of tens of millions of dollars! We often speak of the ‘cost’ of antibiotic resistance to the bacterium, but this is real cost in real time.
To the pharmaceutical industry, the ceaseless, losing battle with microbes has changed from disillusionment over the economics of antibiotic discovery and development to virtual resignation (8). The cost of drug discovery and the stringent Food and Drug Administration requirements in the clinical trial process have increased significantly, while the success rate of discovery has gone down. It has proven ever more difficult to find novel, active compounds with the desired characteristics for use as antibiotics. Even if a potent new compound were to be discovered, it most likely will not be applied in general therapy but will be put on the reserve list for serious, difficult-to-treat infections. There is good reason for this strategy as a means to avoid overuse, thereby limiting resistance development and thus extending the life of the new antibiotic, but limited use also prevents profit! It is difficult for pharmaceutical companies to recoup the expenses of long-term clinical trials; they can more successfully achieve financial gain by producing ‘quality-of-life’ drugs. At the present time, the many reasons for the pharmaceutical industry’s disinterest in antibiotic discovery () are cause for great concern among members of the infectious diseases community. A number of proposals have been made to encourage a variety of government-led actions to reinstate antibiotic discovery programs and to seek viable alternatives to antibiotics (9,10).
At the present time, the many reasons for the pharmaceutical industry’s disinterest in antibiotic discovery () are cause for great concern among members of the infectious diseases community. A number of proposals have been made to encourage a variety of government-led actions to reinstate antibiotic discovery programs and to seek viable alternatives to antibiotics (9,10).
TABLE 3
Why the pharmaceutical industry is abandoning antibiotics
| • | No new antibiotics have been found by traditional approaches. |
| • | High-throughput screening/genomic approaches have been a |
| scientific failure and a financial disaster. | |
| • | Combinatorial chemistry libraries are limited sources of chemical diversity.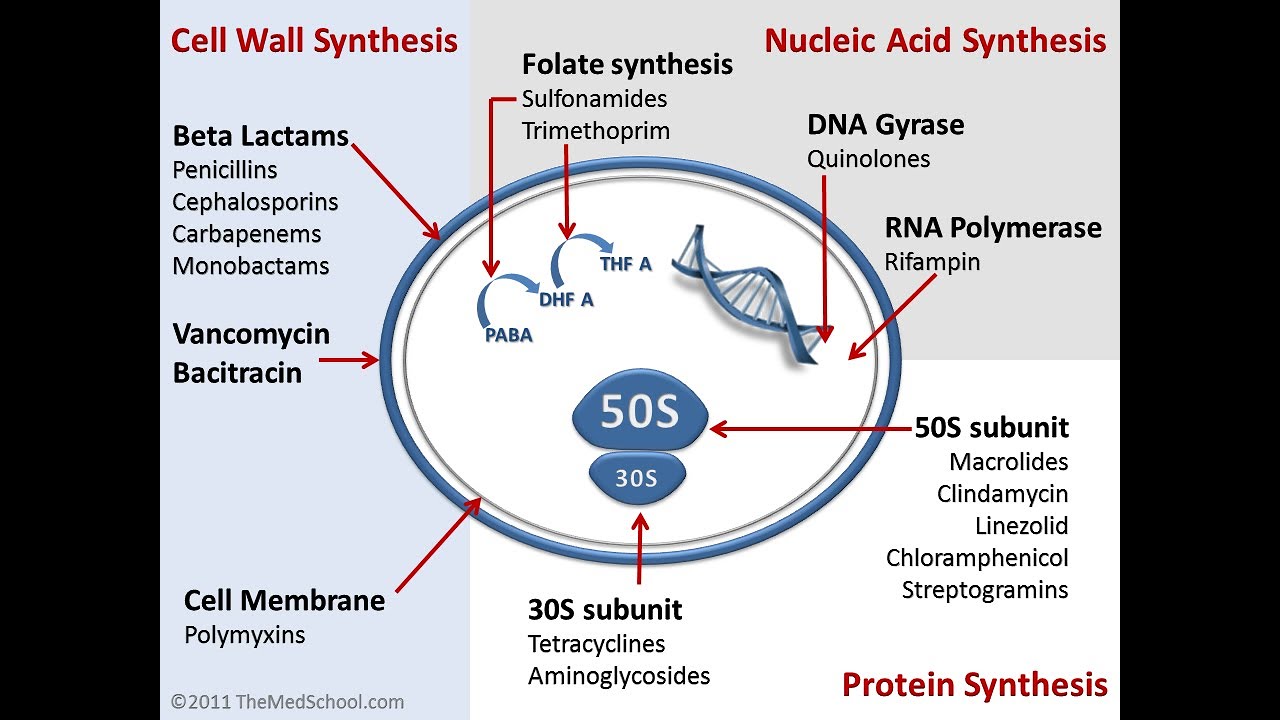 |
| • | It takes too much money and too much time to find antibiotics. |
| • | Are new antibiotics really needed when the old ones still work (mostly)? |
| • | Food and Drug Administration approval is arduous and risky. |
| • | Novel antibiotics are restricted to last-resort use (low sales). |
| • | Quality-of-life ‘drugs’ are more marketable and more profitable; only four |
| antibiotics among 290 agents are currently in clinical development. | |
| • | There is no academic discovery pipeline because of insufficient funding. |
| • | The threat of litigation.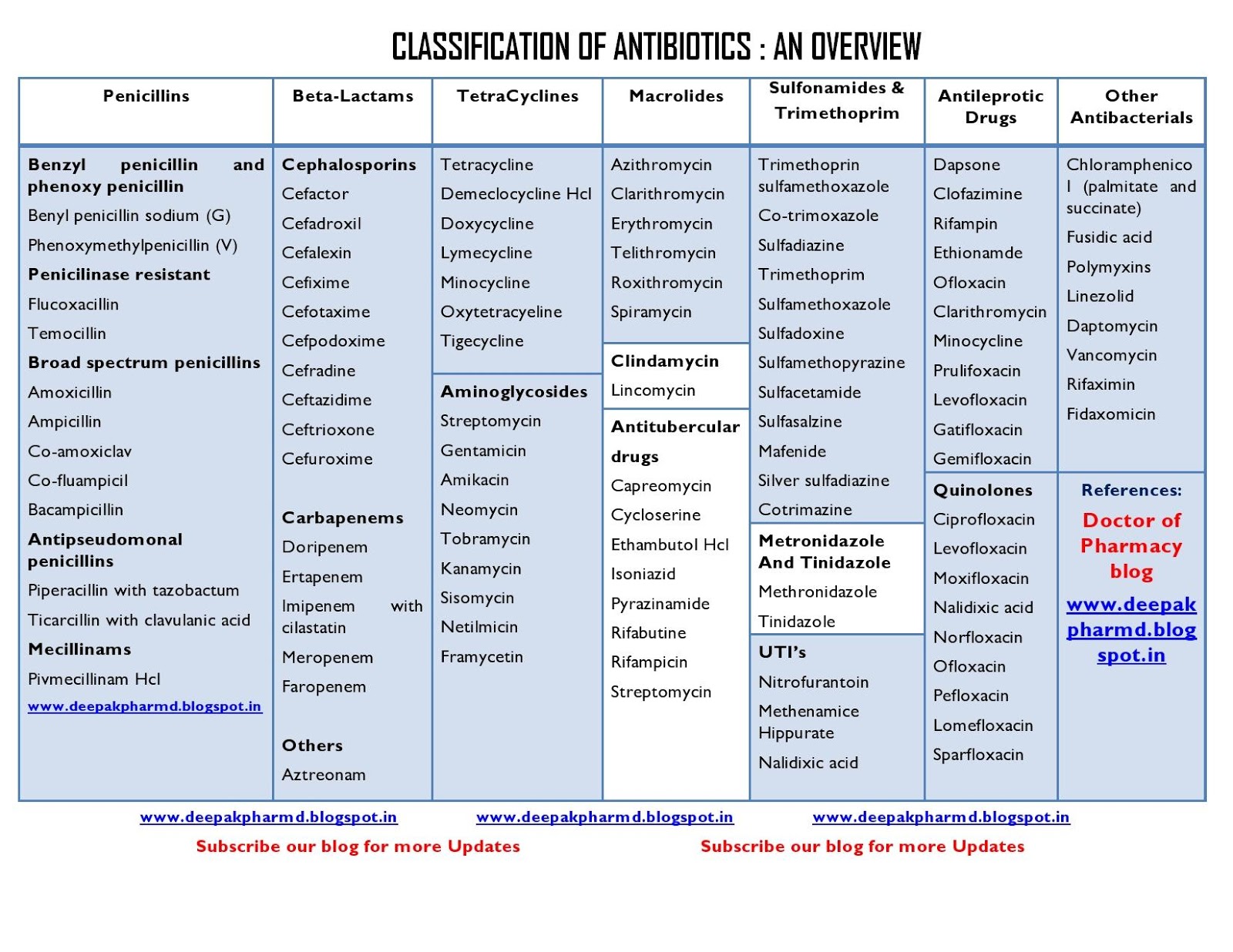 |
Open in a separate window
Nonetheless, antibiotics are an irreplaceable component in the control and treatment of infectious diseases; we cannot do without them. Nature has provided the majority of effective treatments available at this time. But there are millions of bioactive small molecules made by bacteria and fungi awaiting discovery; they have gone through millennia of natural, evolutionary selection to target specific cellular components. This microbial world has barely been mined, mainly because a large proportion of the organisms cannot be grown in the laboratory. However, using modern molecular genetic procedures (such as metagenomic techniques) as a new approach to drug discovery (11), novel microbial strains with novel antibiotic-producing potential can be found, and more and more ‘unculturable’ microbes can be grown in the laboratory. Accessing the rich reservoirs of bioactive microbial products, together with advances in the technology of structure identification, should bring about rapid changes in this field of discovery (12). New antibiotics and other therapeutics will be found.
New antibiotics and other therapeutics will be found.
One important problem remains: will we be able to use these new medicinals in an effective manner so as to preserve their value for longer periods of time? Or will overuse, overprescription and misuse continually plague their application? Numerous suggestions for appropriate antibiotic use have been made, and with diligence, it should be possible to extend the useful lifetimes of antibacterial and other drugs; alternatives, such as vaccines, must be sought (). Several European countries have applied restrictions to the use of antibiotics as animal growth promotants, a policy that has been shown to work: resistance levels in both animal and human populations have declined (13). It is evident that different strategies must be applied and combined to arrive at effective solutions.
TABLE 4
Avoiding and overcoming antibiotic resistance
| • | Optimal use of all antimicrobials through selection, cycling, combination |
| and restriction | |
| • | Novel antimicrobials and their prudent use |
| • | Alternative approaches (immunity, phage, probiotics) |
| • | Better understanding of pathogen, commensal and host biology |
| • | Increased surveillance and epidemiology of resistance |
| • | Improved public and health care specialist education |
| • | Improved hygiene (back to Semmelweis) |
| • | Banning of nontherapeutic uses of antimicrobials |
| • | Reduction in bactericide use |
Open in a separate window
A final question: it is generally accepted that antibiotics are overprescribed, but are they also being misused in a biological sense? We have been using antibiotics for over half a century without having a clue as to their biology and roles in microbial ecology.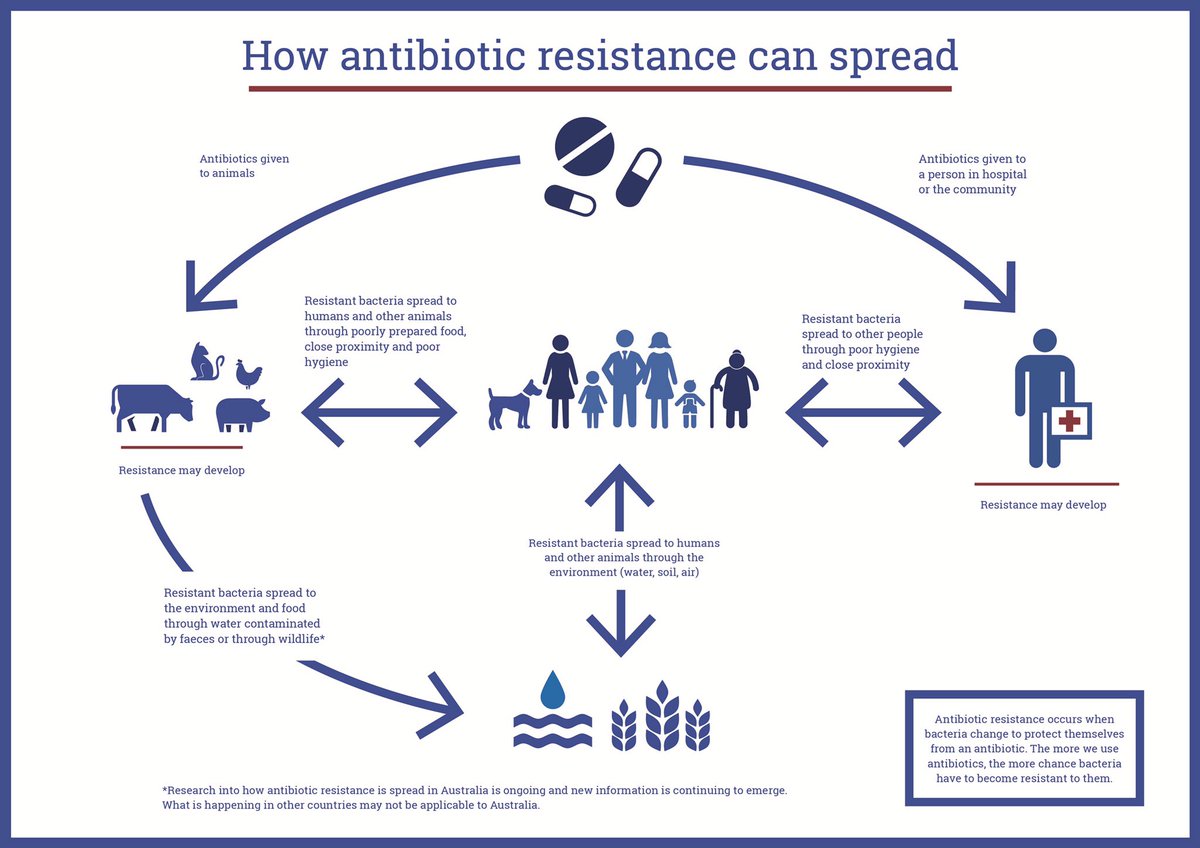 If more research was devoted to studying their natural functions, we may be better equipped to discover new antibiotics and use them to their best advantage in health care.
If more research was devoted to studying their natural functions, we may be better equipped to discover new antibiotics and use them to their best advantage in health care.
The author thanks Dorothy Davies for her help in preparing this paper. Financial support for work performed in the author’s laboratory was provided by the Canadian Institute for Health Research and the Canadian Bacterial Diseases Network.
1. Rowe-Magnus DA, Mazel D. Integrons: Natural tools for bacterial genome evolution. Curr Opin Microbiol 2001;4:565-9. [PubMed] [Google Scholar]
2. Fluit AC, Schmitz FJ. Resistance integrons and super-integrons. Clin Microbiol Infect 2004;10:272-88. [PubMed] [Google Scholar]
3. Skurray RA, Firth N. Molecular evolution of multiply- antibiotic-resistant staphylococci. Ciba Found Symp 1997;207:167-83. [PubMed] [Google Scholar]
4. Fournier P-E, Vallenet D, Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2006;2:e7. [PMC free article] [PubMed] [Google Scholar]
[PMC free article] [PubMed] [Google Scholar]
5. D’Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science 2006;311:374-7. [PubMed] [Google Scholar]
6. Parsek MR, Fuqua C. Biofilms 2003: Emerging themes and challenges in studies of surface-associated microbial life. J Bacteriol 2004;186:4427-40. [PMC free article] [PubMed] [Google Scholar]
7. Jacoby G, Bush K. Beta-lactam resistance in the 21st century. In: White DG, Alekshun MN, McDermott PF, eds. Frontiers in Antimicrobial Resistance. Washington, DC: ASM Press, 2005:53-65. [Google Scholar]
8. Projan SJ. Why is big pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 2003;6:427-30. [PubMed] [Google Scholar]
9. Nathan C. Antibiotics at the crossroads. Nature 2004;431:899-902. [PubMed] [Google Scholar]
10. Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 2006;42:657-68. [PubMed] [Google Scholar]
Clin Infect Dis 2006;42:657-68. [PubMed] [Google Scholar]
11. Gillespie DE, Brady SF, Bettermann AD, et al. Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl Environ Microbiol 2002;68:4301-6. [PMC free article] [PubMed] [Google Scholar]
12. Clardy J, Walsh C. Lessons from natural molecules. Nature 2004;432:829-37. [PubMed] [Google Scholar]
13. Wegener HC. Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol 2003;6:439-45. [PubMed] [Google Scholar]
Antibiotics:
May 11, 2020
Mankind invented antibiotics more than nine decades ago. And this is considered one of the most significant discoveries in world history. However, in recent years, antibacterial drugs have increasingly become the subject of discussion. Some of their participants hold the view that antibiotics are essential medicines that help fight diseases. Other experts are convinced that taking antibacterial drugs is dangerous, because they themselves cause serious disorders in the human body. So what are antibiotics – “friends” or “enemies”? The answers to this and some other questions regarding antibacterial drugs were given by the head of the Department of Military Field Surgery of the Military Medical Faculty at the Belarusian State Medical University, Candidate of Medical Sciences, Associate Professor, Colonel of the Medical Service Dmitry Aleksandrovich Klyuyko.
So what are antibiotics – “friends” or “enemies”? The answers to this and some other questions regarding antibacterial drugs were given by the head of the Department of Military Field Surgery of the Military Medical Faculty at the Belarusian State Medical University, Candidate of Medical Sciences, Associate Professor, Colonel of the Medical Service Dmitry Aleksandrovich Klyuyko.
– Dmitry Alexandrovich, please tell us what antibiotics are? What diseases are they used to treat?
– Translated into Russian, the word “antibiotic” ( lat . anti “against” and Greek bios “life”) means “against life”. In fact, antibacterial drugs were created to inhibit the reproduction and growth of microorganisms, which are pathogenic bacteria. Nowadays, antibacterial drugs defeat serious diseases, many of which were previously considered incurable, such as pneumonia, tuberculosis, gastrointestinal infections, postoperative complications, and some others.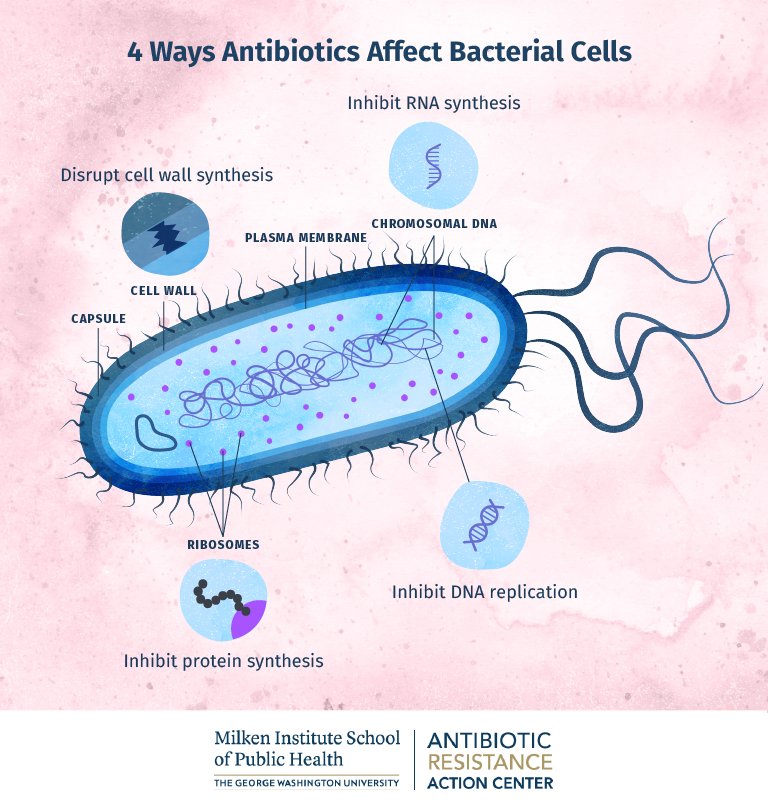
However, antibiotics are absolutely ineffective in viral infections. For example, they will not help with influenza, SARS, hepatitis A, B and C. However, if the flu leads to complications, including pneumonia, the doctor prescribes an antibacterial drug to treat a bacterial infection that has joined the viral disease.
– How many antibiotics are there in the world today?
– Today, several thousand different natural and synthetic substances are known that are used as antibiotics. They are combined into 17 classes, and each of them acts on a certain type of pathogen. Thus, penicillin was the first antibiotic discovered in 1928 by the British microbiologist Alexander Fleming, belongs to the class of beta-lactam antibiotics. Of the known antibacterial drugs, only a small part of them is used – no more than 5%, since most of the previously discovered antibacterial drugs have become useless due to antibiotic resistance.
– What are the different ways of administering antibacterial drugs?
– Distinguish between intravenous, intramuscular and enteral routes of administration of antibiotics. The dosage, number of doses and bioavailability of antibacterial drugs, respectively, depend on them. Each of these methods has its own advantages. So, the drug that enters the body intravenously will act the fastest, then intramuscularly, the slowest enterally.
– What rules should be followed when taking antibacterial drugs?
– Since any antibiotic is a toxic substance, the most important thing is to take care that its use brings maximum benefit and minimum harm. To do this, regardless of the type of antibacterial drugs prescribed, it is advisable to follow the following recommendations.
The first rule of is that antibacterial drugs must be used strictly for their intended purpose and only for such diseases where they are really necessary. Even if this is a “cover” for possible complications, it should be adequate according to all the principles of rationality – a certain dosage, frequency of administration, etc. Otherwise, nothing good, except for harm to the body, should be expected.
Even if this is a “cover” for possible complications, it should be adequate according to all the principles of rationality – a certain dosage, frequency of administration, etc. Otherwise, nothing good, except for harm to the body, should be expected.
Antibacterial drugs as a preventive measure are prescribed for patients with reduced immunity, in the presence of high fever, “bad” cough and similar symptoms, as well as for those who are being treated in a hospital environment where there is a bacterial environment (nosocomial infection). If the patient is treated at home, uses rational anti-inflammatory therapy, which brings positive results, one should not “hide behind” antibiotics.
The second rule – even if the condition improved immediately, the course of admission must be brought to its logical conclusion. For bacteria that are not completely suppressed form resistance to the antibiotic, and further treatment will be ineffective. The minimum duration of treatment with an antibacterial drug is seven days.
The third rule of is that the prescribed dosage must be adequate. Sometimes, in order to avoid side effects, the fight against the disease begins with a small dosage of antibacterial drugs. As a result, it turns out to be ineffective, and soon the bacteria stop responding to the drug and even the class to which it belongs.
Fourth rule – antibiotics should be prescribed by status. That is, if the patient has a strong infection, he must take a potent antibacterial drug, and if it is weak, then the appropriate drug should be taken. And by no means the other way around. In general, antibiotics are prescribed empirically – the doctor assumes that the infection will be hit by the prescribed drug.
Fifth rule – antibiotics do not “work” when there is a source of infection in the body. Therefore, at first it is eliminated, and only then antibacterial drugs are prescribed.
The result of treatment will also be null if the patient’s body does not have enough protein, which is the carrier of antibiotic components.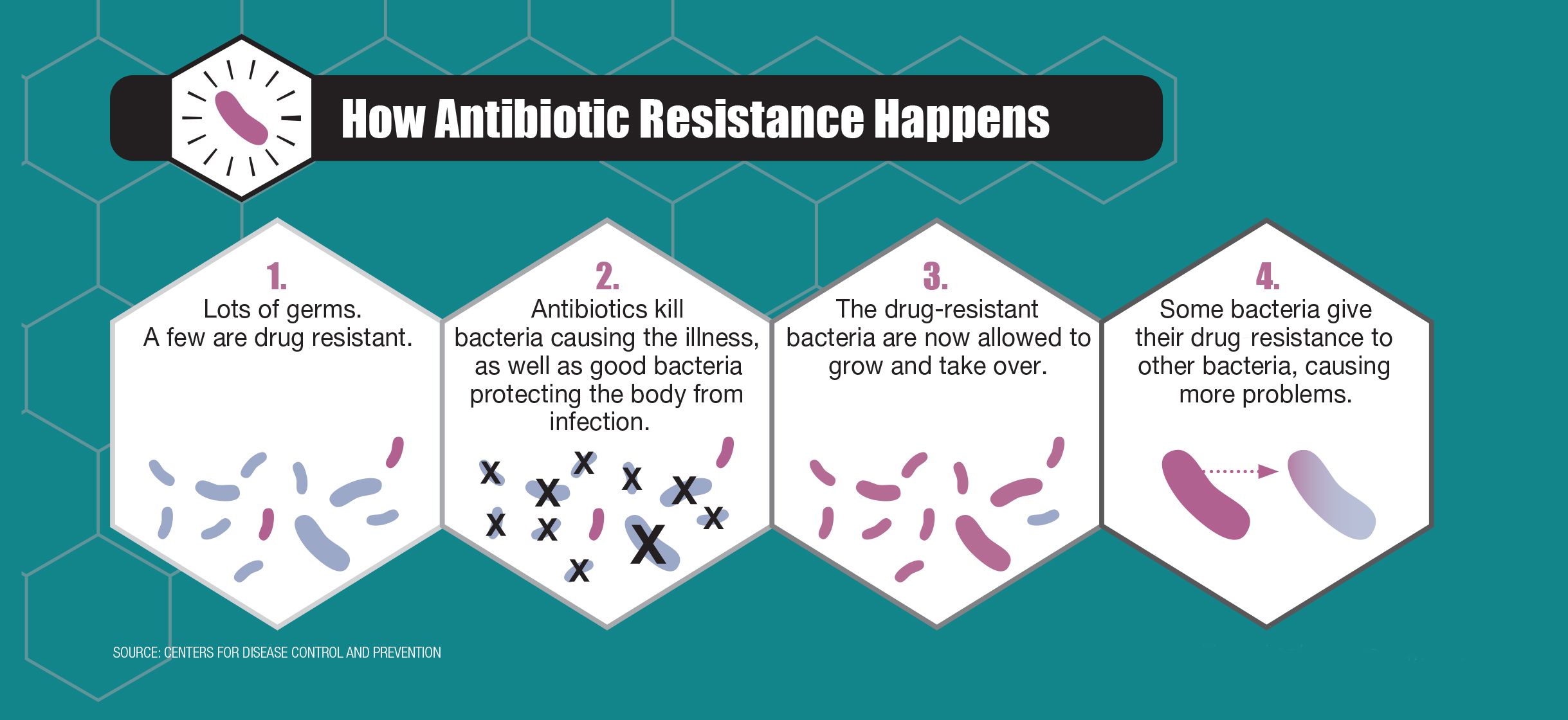 The only way in this case is to replenish protein reserves.
The only way in this case is to replenish protein reserves.
Sixth rule – the same antibiotic can be produced in low and high dosages. This means that you should be careful and purchase it in doses strictly prescribed by your doctor.
Antibiotic treatment is effective only if taken correctly and following all doctor’s prescriptions. In no case should you prescribe antibacterial drugs for yourself, so as not to harm even more.
– What is resistance and why does it occur?
– Resistance is the resistance of microorganisms to antibiotics. The fact is that microorganisms have the ability to evolve. And sooner or later, as a result of mutations, they adapt to the action of one or another antibiotic, which becomes harmless to them. Medical professionals are currently concerned about the fact that pathogenic bacteria adapt to antibiotics much faster than new drugs are invented.
Many doctors talk about the danger of mass use of antibiotics, because due to the rapid pace of development of bacteria, there is a threat of the emergence of resistant flora, which cannot be resisted by modern antibacterial drugs. The first antibiotics (penicillin, biomycin) were of natural origin, they were obtained from mold fungi. They had a narrow spectrum of action and did not harm the body, because its microflora was already adapted to the substances they contain.
The first antibiotics (penicillin, biomycin) were of natural origin, they were obtained from mold fungi. They had a narrow spectrum of action and did not harm the body, because its microflora was already adapted to the substances they contain.
Antibiotics of the new generation are synthetic. Possessing the widest spectrum of action, they kill almost all bacteria, including the beneficial intestinal microflora. At the same time, the pathogenic microflora very quickly adapts to such drugs – in almost 2–3 months, new strains resistant to these antibacterial agents appear. The natural microflora, which is an integral part of our immunity, recovers much more slowly. As a result, immunity drops sharply, and many dangerous pathogens can easily enter the body, as they say, with all the ensuing consequences.
The primary responsibility lies with the patients. If a person took an antibiotic exactly as prescribed by a doctor until complete recovery, there would be no pathogens left in his body. Unfortunately, patients often “drink” antibiotics without a doctor’s instructions, in insufficient concentrations, do not complete the course of treatment, so some of the pathogens survive after such therapy, and their carrier remains contagious to others, even if temporarily does not feel unwell. Doctors, who sometimes prescribe antibacterial drugs unnecessarily, also have a certain share of guilt. As a result, this leads to an increase in the number of microorganisms that simple antibiotics cannot neutralize.
Unfortunately, patients often “drink” antibiotics without a doctor’s instructions, in insufficient concentrations, do not complete the course of treatment, so some of the pathogens survive after such therapy, and their carrier remains contagious to others, even if temporarily does not feel unwell. Doctors, who sometimes prescribe antibacterial drugs unnecessarily, also have a certain share of guilt. As a result, this leads to an increase in the number of microorganisms that simple antibiotics cannot neutralize.
– How can the negative impact of antibiotics on the human body be minimized?
– Antibiotics are designed to aggressively interfere with the vital activity of microorganisms. The targeting accuracy of the effects of drugs on pathogenic bacteria has not yet been achieved, although scientists have been working on this for many years. Therefore, the use of antimicrobial agents is accompanied by side effects and can adversely affect the health and well-being of the patient. In this regard, before using the medicine, it is mandatory to study the instructions. In the presence of diseases indicated in the list of contraindications, you should contact your doctor for advice.
In this regard, before using the medicine, it is mandatory to study the instructions. In the presence of diseases indicated in the list of contraindications, you should contact your doctor for advice.
Do not take the drug on an empty stomach, so as not to increase mucosal irritation. Antibiotics must be taken with water. In this case, the use of alcohol, absorbent and blood-thinning drugs should be excluded.
To maintain normal intestinal microflora, it is recommended to take probiotics, drugs with lactobacilli, immunomodulators and vitamin complexes.
The use of antibiotics in our time is a kind of arms race, when the bacterium dictates its conditions, and medicine is forced to adapt to them and offer antibacterial drugs that meet these requirements. The consequences of the use of such drugs are global in nature, when one or another antibiotic ceases to affect the population. And with each generation, antimicrobial drugs become more toxic. In turn, the bacteria “do not waste time” and adapt to a new enemy, showing even greater aggressiveness. The individual nature of the consequences of the use of antibiotics is that they have a negative effect on the functioning of internal organs. That is why in our country, as well as throughout the civilized world, antibacterial drugs are mostly sold in pharmacies only by prescription.
The individual nature of the consequences of the use of antibiotics is that they have a negative effect on the functioning of internal organs. That is why in our country, as well as throughout the civilized world, antibacterial drugs are mostly sold in pharmacies only by prescription.
To avoid all sorts of diseases, you should strengthen your immune system by adopting a healthy lifestyle, including proper nutrition, physical education, hardening and much more. Antibiotics, in which case, should be the last line of treatment.
Interviewed by Oksana Kurbeko, press secretary,
photo from the personal archive of Dmitry Klyuiko and open sources
0001
We were told from childhood that matches are not toys. We would add many other things to the same row with matches, including antibiotics.
Uncontrolled and widespread use of antibiotics for all kinds of coughs or snot, it turns out, can lead to unexpected consequences.
Danil Sergeevich Simanovsky, pediatrician, chief physician of the Osnova Deti children’s clinic, answers questions about antibiotics.
Why doesn’t the doctor prescribe antibiotics if the child has a severe cold?
There is no diagnosis of a cold. A cold usually refers to the presence of snot, cough, red throat or sore throat – what doctors call catarrhal phenomena. And all these phenomena can be caused by viral infections, and can be bacterial. Depending on the etiology of the infection (what caused the infection), antibiotics are used or not.
Even if the snot, cough and red throat are strong enough and bring discomfort, but the infection is caused by a virus, then antibiotics are not prescribed because they are useless, because viruses are not sensitive to antibiotics. Antibiotics were invented and used only against bacteria. Therefore, if a child is sick for a long time and seriously, and you have doubts: is it a common cold, you need to see a doctor. The doctor will examine the child, determine the etiology of the infection, sometimes additional studies are needed for this: blood tests, smears. Only then will prescribe antibiotics if necessary.
Only then will prescribe antibiotics if necessary.
If yellow or green mucus comes out of the nose, is this a sign of a bacterial infection?
It is impossible to determine the etiology of the infection by the color of the snot. And everyone knows that green snot is scary, but white snot is not. This is not the case, because the mucus that is secreted in the nasopharynx can take on a color depending on the additional properties of the bacteria. Most often, the green color of the snot acquires from the green staphylococcus aureus, which is the normal flora of the nasopharynx. Remember, the color of the snot has no independent value.
Do I need to take a preparation for the intestinal microflora when taking antibiotics?
The microflora of the large intestine is very mobile, therefore, for a person without gastrointestinal diseases, who receives treatment with an antibacterial drug according to the correct indications, the appointment of probiotics, eubiotics is not a mandatory necessity. But the doctor always looks at the child, additional risks, age and so on. As a rule, when taking antibiotics, we do not observe dysbacteriosis, but diarrhea, which is a side effect of antibiotics and disappears as soon as we finish this therapy. For example, the most common penicillin antibiotic in outpatient practice is amoxicillin, which is most often used in the treatment of otitis media, bronchitis, and tonsillitis. It contains clavulanic acid, which has a natural side effect – diarrhea, i.e. loose stools. If such a side effect from an antibacterial drug occurs, it is enough to take an enterosorbent, but not simultaneously with antibiotics. All at the discretion of the doctor.
But the doctor always looks at the child, additional risks, age and so on. As a rule, when taking antibiotics, we do not observe dysbacteriosis, but diarrhea, which is a side effect of antibiotics and disappears as soon as we finish this therapy. For example, the most common penicillin antibiotic in outpatient practice is amoxicillin, which is most often used in the treatment of otitis media, bronchitis, and tonsillitis. It contains clavulanic acid, which has a natural side effect – diarrhea, i.e. loose stools. If such a side effect from an antibacterial drug occurs, it is enough to take an enterosorbent, but not simultaneously with antibiotics. All at the discretion of the doctor.
Can antibiotic resistance develop?
Of course it can, and we are very afraid of it. Therefore, the scientific and medical communities are struggling with polypharmacy – the prescription of too many drugs that are inadequate in relation to the disease. It is also very important to combat the self-prescribing of antibiotics by parents. Therefore, it is not necessary to focus on either the color or the smell of snot, or anything else. It is necessary to be guided by the recommendations of the doctor. If the recommendations seem unreasonable to you, you need to seek a second opinion. freepik.com
Therefore, it is not necessary to focus on either the color or the smell of snot, or anything else. It is necessary to be guided by the recommendations of the doctor. If the recommendations seem unreasonable to you, you need to seek a second opinion. freepik.com
More about resistance to antibiotics
Multiresistance is not the resistance of the child’s body to an antibiotic, but the resistance of microorganisms contained in the body. On the mucosa at the same time there is a huge amount of viruses and bacteria, and this is normal. Some of them are pathogenic, some are conditionally pathogenic, some are saprophytes, that is, normal flora. For example, a large number of skin problems arise from the regular use of antibacterial agents. Not only because they dry the skin, but because they disrupt the normal biocenosis of the skin. The same thing happens when you constantly use antibacterial toothpaste or antibacterial intimate hygiene gels. Our everyday behavior already increases the likelihood that microorganisms that live on the mucous membranes and on the skin will develop resistance to these antibacterial agents. And when we need to cure some kind of infection, a situation may arise that the chosen drug will not work. In this case, you will have to choose other antibiotics with a broader spectrum of action in order to still defeat this infection. It is with this that the need to prescribe the correct dose is connected. Do not give an antibiotic just in case for a couple of days. Because when the antibiotic dose is too small, resistance develops: hostile microorganisms are not completely defeated: some die, but the surviving bacteria will already be resistant to this antibiotic. The duration of antibiotic therapy is an important factor. If it is not observed, the likelihood of developing resistance to antibiotics increases.
And when we need to cure some kind of infection, a situation may arise that the chosen drug will not work. In this case, you will have to choose other antibiotics with a broader spectrum of action in order to still defeat this infection. It is with this that the need to prescribe the correct dose is connected. Do not give an antibiotic just in case for a couple of days. Because when the antibiotic dose is too small, resistance develops: hostile microorganisms are not completely defeated: some die, but the surviving bacteria will already be resistant to this antibiotic. The duration of antibiotic therapy is an important factor. If it is not observed, the likelihood of developing resistance to antibiotics increases.
How long does it take for antibiotics to work?
Another question: the effectiveness of antibiotic therapy is assessed based on the results of 48-72 hours from the start of oral antibiotic intake. These hours must be counted from the moment of the first reception. This does not mean that recovery occurs after 2-3 days, but there should be an improvement in both well-being and the symptoms of the disease, which the doctor evaluates during the examination. There are diseases that, from the point of view of parents, are treated inadequately for a long time, for example, tonsillitis. The course of antibiotic treatment is 10 days. By the fourth day, the child is feeling well, he has no complaints, and the doctor insists on continuing to take “a harmful, terrible antibiotic that kills everything.” This is necessary so that the bacteria do not develop resistance, but die from antibiotics. When children come to the appointment, to whom their parents have independently prescribed an antibiotic for a common ARVI, doctors choose the minimum course of taking the medicine. If we cancel them on the second or third day, we risk provoking the resistance of the remaining microorganisms. And next time, we’ll have to use a heavier, broader, and more harmful antibiotic for the baby.
This does not mean that recovery occurs after 2-3 days, but there should be an improvement in both well-being and the symptoms of the disease, which the doctor evaluates during the examination. There are diseases that, from the point of view of parents, are treated inadequately for a long time, for example, tonsillitis. The course of antibiotic treatment is 10 days. By the fourth day, the child is feeling well, he has no complaints, and the doctor insists on continuing to take “a harmful, terrible antibiotic that kills everything.” This is necessary so that the bacteria do not develop resistance, but die from antibiotics. When children come to the appointment, to whom their parents have independently prescribed an antibiotic for a common ARVI, doctors choose the minimum course of taking the medicine. If we cancel them on the second or third day, we risk provoking the resistance of the remaining microorganisms. And next time, we’ll have to use a heavier, broader, and more harmful antibiotic for the baby.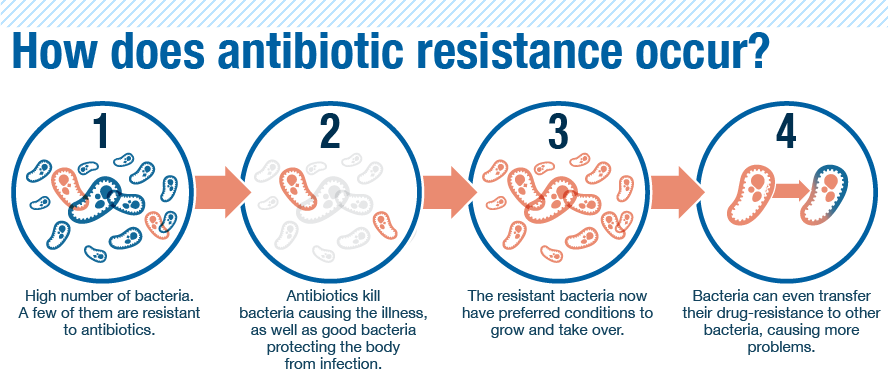
One antibiotic helped the older child, why go to the doctor if the younger child can be given the same one?
If children have the same infection, the same antibiotic will most likely help them. But, at a minimum, it is necessary to correctly determine the dosage of this antibiotic, perhaps your child needs an individually selected dosage. And we, doctors, have the opportunity to deviate a little from the dosages that are written in the annotations of medicines. Because the doctor, unlike the mother, also has regulations in the form of clinical recommendations for treatment. The dosage of the antibiotic will be different for the treatment of different diseases, so even though you have several children sick one after another stereotyped, you still need to consult a doctor.
Doesn’t a cold often turn into a bacterial infection?
Why wait for this when you can immediately take antibiotics? Firstly, it is absolutely scientifically proven that taking antibiotics against the background of a viral infection does not reduce the likelihood of complications.