Milk antibiotics. Milk and Antibiotics: Understanding Interactions and Safe Usage
Why do milk and antibiotics not mix well. How does food affect antibiotic absorption. What are the risks of improper antibiotic use. How can patients ensure effective antibiotic treatment.
The Complex Relationship Between Milk and Antibiotics
The interaction between milk and antibiotics is a crucial topic in healthcare that often raises questions among patients. Understanding why certain antibiotics shouldn’t be taken with milk can help ensure the effectiveness of treatments and prevent potential complications.
Calcium, a primary component in milk, can bind to some antibiotics, particularly tetracyclines. This binding process can significantly reduce the absorption of the antibiotic in the gastrointestinal tract, potentially rendering the medication less effective or even ineffective.
Which antibiotics are affected by milk?
Tetracyclines are the classic family of antibiotics that cannot be taken with milk. However, it’s important to note that not all antibiotics are affected by milk or dairy products. Some common antibiotics that interact with calcium include:
- Doxycycline
- Minocycline
- Tetracycline
For these medications, it’s typically recommended to avoid dairy products for at least 2 hours before and after taking the antibiotic.
Food Interactions with Antibiotics: Beyond Milk
While milk is a well-known interactor with certain antibiotics, various other foods can also influence antibiotic absorption and effectiveness. Understanding these interactions is crucial for optimal treatment outcomes.
How does food affect antibiotic absorption?
The impact of food on antibiotic absorption can vary widely depending on the specific medication. In general, food interactions with antibiotics fall into three categories:
- Decreased absorption: Some antibiotics are less effectively absorbed when taken with food.
- No effect: Certain antibiotics are not significantly impacted by food intake.
- Improved absorption: A few antibiotics actually have better absorption when taken with food.
The relative acidity of the stomach, the presence of fat or other nutrients, and specific elements like calcium can all influence how well an antibiotic is absorbed and delivered to the infected area.

Examples of food-antibiotic interactions
- Penicillins: Generally less affected by food, but acidic foods can reduce absorption of some types.
- Fluoroquinolones: Dairy products, iron supplements, and antacids can reduce absorption.
- Macrolides: Fatty foods can increase absorption of some macrolides like erythromycin.
The Importance of Following Prescription Instructions
Given the complexity of antibiotic-food interactions, it’s crucial to follow the specific instructions provided with your prescription. Pharmacists are experts in these interactions and provide tailored advice for each medication.
Why is it critical to follow antibiotic instructions precisely?
Adhering to prescription instructions ensures that the antibiotic reaches its intended concentration in the bloodstream and at the site of infection. Failure to follow these guidelines may result in:
- Reduced effectiveness of the antibiotic
- Potential treatment failure
- Increased risk of antibiotic resistance
- Unnecessary side effects
If you’re unsure about how to take your antibiotic or have questions about potential interactions, always consult your pharmacist or healthcare provider for clarification.

Antibiotic Resistance: A Growing Concern
The improper use of antibiotics has led to a significant global health issue: antibiotic resistance. This phenomenon occurs when bacteria evolve to withstand the effects of antibiotics, making infections harder to treat.
How does antibiotic resistance develop?
Antibiotic resistance can develop through several mechanisms:
- Overuse of antibiotics
- Improper use (not completing the full course)
- Use of antibiotics for viral infections (which they cannot treat)
- Agricultural use of antibiotics in livestock
When antibiotics are used incorrectly or unnecessarily, it gives bacteria the opportunity to adapt and develop resistance mechanisms. This can lead to the emergence of “superbugs” – bacteria that are resistant to multiple types of antibiotics.
What are the consequences of antibiotic resistance?
The rise of antibiotic-resistant bacteria poses several serious threats:
- Increased difficulty in treating common infections
- Higher healthcare costs due to longer hospital stays and more expensive treatments
- Increased mortality rates from previously treatable infections
- Potential return to a “pre-antibiotic era” for some types of infections
To combat this issue, it’s crucial for both healthcare providers and patients to use antibiotics responsibly and only when necessary.
Special Considerations: Antibiotics and Chronic Conditions
Patients with chronic conditions often face unique challenges when it comes to antibiotic use. These individuals may be more susceptible to infections or have complicating factors that affect antibiotic efficacy.
Catheters and recurrent urinary tract infections
Patients with long-term indwelling catheters, such as those with multiple sclerosis or spinal cord injuries, are at high risk for recurrent urinary tract infections (UTIs). However, the approach to preventing and treating these infections is not straightforward.
Chronic antibiotic use as a preventive measure is generally not recommended in these cases due to several factors:
- Risk of developing antibiotic-resistant bacteria
- Ineffectiveness in eradicating bacterial colonization of the catheter
- Potential for more severe infections caused by resistant organisms
Instead, management typically involves:
- Regular catheter changes
- Proper hygiene and catheter care
- Treating symptomatic infections promptly
- Considering alternative methods of bladder management when possible
Kidney stones and antibiotic treatment
The presence of kidney stones can complicate UTI treatment and prevention. Some kidney stones, known as struvite or infection stones, are directly related to certain types of bacterial infections.

These stones form when bacteria that produce the enzyme urease are present in the urinary tract. Urease breaks down urea into ammonia, which can combine with magnesium and phosphate to form stones.
In cases of struvite stones:
- Antibiotic therapy alone is usually not effective
- Surgical removal of the stones is often necessary
- Ongoing stone presence can lead to persistent bacterial colonization
Management of these cases typically requires collaboration between urologists and infectious disease specialists to develop an appropriate treatment plan.
Optimizing Antibiotic Therapy: Best Practices
To ensure the most effective antibiotic treatment and minimize the risk of resistance, healthcare providers and patients should follow certain best practices.
For healthcare providers:
- Prescribe antibiotics only when necessary and appropriate
- Choose the narrowest spectrum antibiotic effective against the suspected pathogen
- Prescribe the correct dose and duration of therapy
- Educate patients on proper antibiotic use and potential side effects
- Consider local antibiotic resistance patterns when selecting therapy
For patients:
- Take antibiotics exactly as prescribed
- Complete the full course of antibiotics, even if symptoms improve
- Do not share antibiotics or use leftover antibiotics from previous prescriptions
- Inform healthcare providers of all medications and supplements being taken
- Practice good hygiene to prevent the spread of infections
By following these guidelines, we can maximize the effectiveness of antibiotic treatments while minimizing the risk of resistance development.

The Future of Antibiotic Use and Development
As antibiotic resistance continues to pose challenges, researchers and healthcare professionals are exploring new approaches to combat bacterial infections and preserve the effectiveness of existing antibiotics.
What are some promising areas of research?
Several innovative strategies are being investigated to address the antibiotic resistance crisis:
- Development of new classes of antibiotics
- Combination therapies to overcome resistance mechanisms
- Bacteriophage therapy using viruses that target specific bacteria
- Immunotherapies to boost the body’s natural defenses against infections
- Nanotechnology-based delivery systems for more targeted antibiotic delivery
These approaches hold promise for expanding our arsenal against bacterial infections and potentially overcoming current resistance challenges.
How can we preserve the effectiveness of current antibiotics?
Preserving the efficacy of existing antibiotics is crucial. Strategies to achieve this include:

- Implementing antibiotic stewardship programs in healthcare settings
- Improving diagnostic tools to quickly identify bacterial infections and their susceptibilities
- Reducing antibiotic use in agriculture
- Enhancing public education about appropriate antibiotic use
- Developing new economic models to incentivize antibiotic development
By combining these efforts with ongoing research and development, we can work towards a future where antibiotics remain effective tools in fighting bacterial infections.
Conclusion: Balancing Antibiotic Use and Health
The relationship between antibiotics, food, and our health is complex and multifaceted. While antibiotics are crucial tools in fighting bacterial infections, their effectiveness can be compromised by improper use or interactions with substances like milk.
By understanding these interactions, following prescription instructions carefully, and using antibiotics responsibly, we can help ensure that these life-saving medications remain effective for future generations. As research continues to advance our understanding of antibiotics and bacterial resistance, it’s crucial that both healthcare providers and patients stay informed and committed to optimal antibiotic use practices.
Remember, the key to effective antibiotic use lies in a partnership between healthcare providers and patients. By working together, asking questions, and following expert guidance, we can maximize the benefits of antibiotic therapy while minimizing risks and preserving these vital medications for the future.
Why Milk and Antibiotics Don’t Mix – Healthy Living Center
Q1. Why is taking antibiotics with milk a no-no? Does this apply to all antibiotics, or only certain ones?
— Shirley, Ohio
It’s not just milk — there are many other foods that can interfere with antibiotics, as well as other drugs.
In order for oral antibiotics to be effective, they must be absorbed from the gastrointestinal tract, make their way into the bloodstream, and be delivered to the infected area. Many factors influence the body’s ability to accomplish this feat, including the relative acidity of the stomach, the presence of fat or other nutrients in the stomach, and whether certain elements such as calcium are present. The classic family of antibiotics that cannot be taken with milk are the tetracyclines, because the calcium in the milk binds the antibiotic and prevents gut absorption.
For most antibiotics, food results in either a decrease in absorption or has no effect. However, some antibiotics are actually better absorbed when taken with food, and it is recommended that others be taken while eating, because the food does not have a significant impact on absorption and may decrease any potential stomach upset from the drugs.
However, some antibiotics are actually better absorbed when taken with food, and it is recommended that others be taken while eating, because the food does not have a significant impact on absorption and may decrease any potential stomach upset from the drugs.
It is very important to follow the directions on the prescription bottle, because pharmacists are the experts in these interactions. Not following directions may result in the antibiotic failing to cure the infection.
Q2. My mom is 64 and has had multiple sclerosis for over 30 years. She is totally immobile and has been on a catheter for about one year. She has a problem with kidney stones and recurring urinary tract infections. Is there any way she could take some kind of antibiotic as a preventive measure to keep her from getting these infections so frequently?
Your mother’s situation is complicated. Anyone with an indwelling catheter in the bladder will develop chronic colonization of the urinary tract by bacteria. There is no good way to eradicate such colonization. Furthermore, chronic use of antibiotics in such situations can lead to the development of resistant organisms, which can lead to more severe infections.
There is no good way to eradicate such colonization. Furthermore, chronic use of antibiotics in such situations can lead to the development of resistant organisms, which can lead to more severe infections.
A complicating factor is the presence of kidney stones. Some kidney stones are related to the presence of bacteria. Certain bacteria contain an enzyme that breaks down urea, a normal component of the urine. The breakdown product is ammonium, a compound that, in combination with magnesium and phosphate, forms stones. These are often called “infection stones” or “struvite stones.” Once these struvite stones form, they can be eradicated only by surgical treatment. Antibiotic therapy is not effective. Furthermore, until the stones are removed, there will always be an additional bacterial load in the urine.
Your mother’s problem requires the input of a specialist. If you mother is not being cared for by a urologist, I suggest that you seek one out. Urologists are surgeons who treat both medical and surgical aspects of kidney stone disease and bladder infection and can work with you and your mother to develop a treatment plan.
Learn more in the Everyday Health Healthy Living Center.
5 Ways Your Pharmacist Can Help You Save Money
Asking the right questions and knowing what to look for can help save money on prescription drug costs.
By Debra Fulghum Bruce, PhD
The 6 Best Over-the-Counter Hearing Aids to Buy in 2023
Looking for the best over-the-counter hearing aid brands? Read our review to find top models, features, and prices on Jabra Enhance, Lexie, Audien, Eargo…
By Cara Everett
The 7 Most Affordable Hearing Aids in 2023
Looking for the best cheap hearing aids? Read our review of top affordable hearing aids. Best OTC and prescription brands include Jabra Enhance, Lexie…
By Cara Everett
Rechargeable Hearing Aids: Are They Right for You?
Rechargeable hearing aids promise convenience, long-lasting performance, and savings on disposable batteries. But are they right for you?
But are they right for you?
By Jennifer Walker-Journey
‘Stuck’ Stem Cells Appear to Cause Graying Hair
Discovering what causes hair to go gray may one day help researchers figure out how to reverse gray hair and even hair loss.
By Kaitlin Sullivan
Using medication: Using antibiotics correctly and avoiding resistance – InformedHealth.org
Created: November 14, 2008; Last Update: December 18, 2013; Next update: 2020.
The development of antibiotics was one of the great discoveries in modern medicine. They fight bacteria and can cure life-threatening infectious diseases such as pneumonia, for which there was previously no effective treatment. But the improper use of antibiotics means that more and more bacteria are becoming resistant to this kind of medication. So it is especially important to use them correctly.
Antibiotics can save lives, but they also relieve symptoms of bacterial infections and help us recover faster. But treatment with antibiotics also has side effects. Nausea or diarrhea are common, for example.
But treatment with antibiotics also has side effects. Nausea or diarrhea are common, for example.
Antibiotics are also used far too often, and improper use is widespread. This has caused many different types of bacteria to become resistant (unresponsive) to antibiotics. Because resistance has become more common, many diseases cannot be treated as well as they could in the past.
When using antibiotics, it’s important to know the following things to prevent resistance and side effects:
Antibiotics only work against bacteria. Many infections are caused by viruses and can’t be treated using antibiotics – examples include respiratory illnesses such as a cough, stuffy nose, bronchitis or the flu.
Excessive and improper use of antibiotics causes side effects, and in the long term reduces their effectiveness.
What is antibiotic resistance?
In medicine, bacteria and other germs are said to be resistant if they are especially able to withstand exposure to external influences. For example, most germs that enter the stomach with food will be killed by stomach (gastric) acid. But some bacteria are covered with a mucous coating that protects them from the acid. They are resistant to gastric acid.
For example, most germs that enter the stomach with food will be killed by stomach (gastric) acid. But some bacteria are covered with a mucous coating that protects them from the acid. They are resistant to gastric acid.
Resistance to antibiotics works on a similar principle: The bacteria have acquired a new property that protects them from the antibiotic. Some types of bacteria can produce a substance that makes certain antibiotics ineffective, for example. Bacteria that can protect themselves from several different antibiotics are referred to as “multiresistant.”
What causes resistance?
Many of the bacteria that are now resistant used to be sensitive to antibiotics. There are a few developments that played a role in this. To put it briefly, one kind of antibiotic could originally neutralize a certain type of bacteria and then effectively stop the infection. But the genetic material of bacteria can change by chance, sometimes creating new properties. If they protect the bacteria from an antibiotic, then the bacteria have become resistant. These kinds of properties can also transfer from one type of bacteria to another.
These kinds of properties can also transfer from one type of bacteria to another.
If antibiotics are used very often, resistant bacteria are better able to reproduce because the other non-resistant strains of bacteria are stopped. Antibiotics then no longer help against infections caused by resistant bacteria.
Which bacteria are resistant to antibiotics and why are they dangerous?
Strains of Streptococcus and Staphylococcus bacteria are often resistant to antibiotics. One example is called “methicillin-resistant Staphylococcus aureus” (MRSA). Staphylococci can be found on skin and mucous membranes and may cause infection – for example if they get into open wounds.
Resistant strains have now developed in other types of bacteria, such as Escherichia coli, Klebsiella and pseudomonads.
What is being done about antibiotic resistance?
In Germany, antibiotics are prescription-only. This means that doctors are first and foremost responsible for careful and appropriate use. They are to first see whether someone actually has a bacterial infection. If they do, then it’s important that the antibiotic is prescribed at the right dose and for long enough, and that the right antibiotic is selected that will most effectively fight the bacteria.
They are to first see whether someone actually has a bacterial infection. If they do, then it’s important that the antibiotic is prescribed at the right dose and for long enough, and that the right antibiotic is selected that will most effectively fight the bacteria.
There are also hygiene regulations to keep resistant bacteria from spreading further and preventable infections from occurring. These measures are especially important inside of a hospital. Antibiotics are used there relatively frequently, so resistant germs can develop quite quickly. If you come into contact with someone who has an infection of resistant bacteria, it can help to wear disposable gloves, a mask and coat, and to use a hand disinfectant to stop the spread of the germs.
Antibiotics are also used in veterinary medicine and in agriculture. Veterinarians also have to comply with the rules for handling antibiotics properly.
What can I do to prevent antibiotic resistance?
Being cautious when taking antibiotics can help prevent both antibiotic resistance and side effects.
The most important thing is to not overestimate what antibiotics can do: Patients often expect antibiotics to be prescribed to treat medical conditions for which they are not suitable.
Antibiotics are needed to treat serious bacterial infections like lung infections or meningitis (inflammation of the membranes lining the brain and spinal cord). This is not the case when, for example, people who are otherwise healthy have respiratory infections caused by viruses, such as a cold or influenza (“the flu”). Antibiotics will usually be of no help because they only fight bacteria. Antibiotics also have side effects including allergic reactions, stomach and bowel problems, nausea and fungal infections. Because of these associated risks, it’s important to carefully consider the advantages and disadvantages of taking antibiotics.
What’s important to consider when taking antibiotics?
Antibiotics should be taken for as long as the doctor has prescribed them. Just because the symptoms of the illness subside, it doesn’t mean that all of the germs have been killed. Remaining bacteria may cause the illness to start up again.
Remaining bacteria may cause the illness to start up again.
If there are some tablets left over, they should not be kept for later use or given to other people. Leftover medication can be disposed of in the normal garbage or dropped off at some pharmacies. Pharmacies are not obligated to accept opened medicine though. It is important not to dispose of the medication by pouring it down the drain or flushing it down the toilet. That is bad for the environment and also contributes to bacterial resistance.
Medications can only work properly if they are used correctly. It’s important to know the following things when taking antibiotics:
Can the tablets be broken into smaller pieces to make them easier to swallow? Doing this can stop some medications from working properly.
What food can you take antibiotics with? Antibiotics are usually taken with water because taking them together with fruit juices, dairy products or alcohol can affect how the body absorbs some drugs.
 Dairy products include milk as well as butter, yogurt, and cheese. After taking an antibiotic you may need to wait for up to three hours before eating or drinking any dairy products. Grapefruit juice and dietary supplements containing minerals like calcium may also work dampen the effect of antibiotics.
Dairy products include milk as well as butter, yogurt, and cheese. After taking an antibiotic you may need to wait for up to three hours before eating or drinking any dairy products. Grapefruit juice and dietary supplements containing minerals like calcium may also work dampen the effect of antibiotics.When should you take antibiotics? Some antibiotics are always meant to be taken at the same time of day, others are meant to be taken before, with or after a meal. If you are supposed to take the medicine three times a day, for example, it usually needs to be taken at set times so that the effect is spread out evenly over the course of the day. You could remember the regular times of 6 a.m., 2 p.m. and 10 p.m. for an antibiotic that needs to be taken every 8 hours, for example.
Can you take antibiotics together with other medications? Because antibiotics can interact with other medications, it’s important to tell your doctor if you take other medications too.
 Antibiotics might interact with some blood thinners and antacids, for example. Some antibiotics can make birth control pills less effective.
Antibiotics might interact with some blood thinners and antacids, for example. Some antibiotics can make birth control pills less effective.
You can find detailed information on the use of a specific antibiotic in the package insert. If you’re not sure about what is important to consider when taking the antibiotic, you can ask your doctor or pharmacist.
Sources
Bundesministerium für Gesundheit (BMG), Bundesministerium für Ernährung und Landwirtschaft (BMEL), Bundesministerium für Bildung und Forschung (BMBF). DART 2020. Zwischenbericht anlässlich der WHA 2016. May 2016.
Centers for Disease Control and Prevention (CDC). Antibiotic / Antimicrobial Resistance. June 12, 2017.
Deutsche Gesellschaft für Infektiologie e.V. (DGI). S3-Leitlinie: Strategien zur Sicherung rationaler Antibiotika-Anwendung im Krankenhaus. AWMF-Register-Nr.: 092-001. December 15, 2013.
Kayser FH, Böttger EC, Deplazes P, Haller O, Roers A.
 Taschenlehrbuch Medizinische Mikrobiologie. Stuttgart: Thieme; 2014.
Taschenlehrbuch Medizinische Mikrobiologie. Stuttgart: Thieme; 2014.Weltgesundheitsorganisation (WHO). Antimicrobial resistance. October 2016.
IQWiG health information is written with the aim of helping
people understand the advantages and disadvantages of the main treatment options and health
care services.Because IQWiG is a German institute, some of the information provided here is specific to the
German health care system. The suitability of any of the described options in an individual
case can be determined by talking to a doctor. We do not offer individual consultations.Our information is based on the results of good-quality studies. It is written by a
team of
health care professionals, scientists and editors, and reviewed by external experts. You can
find a detailed description of how our health information is produced and updated in
our methods.
Antibiotics in the dairy industry: what everyone should know
Presentation by Vitaly Bashinsky, FAO Specialist (Food and Agriculture Organization of the United Nations, FAO UN) on veterinary medicine and biosafety.
The DairyNews presents the text version of Vitaly Bashinsky’s speech at the conference “The vector of the dairy industry in Kyrgyzstan – a course for development” in Bishkek. The event was organized by the Dairy Union of Kyrgyzstan with the support of the UN FAO and the EBRD.
Six myths about antibiotics in milk
Myth No. 1. Small doses of antibiotics are not harmful, but even slightly beneficial.
It’s a lie. Small doses of antibiotics do not help us at all, they are harmless to microorganisms, which at the same time get acquainted with our drug and become resistant to it.
Myth #2: Boiling removes antibiotics from milk and meat.
This is partly true. Indeed, if you cook meat and pour out the first broth, then most of the antimicrobial agents will be removed. But there is no way to completely remove them.
Myth #3: Natural antibiotics are safer than synthetic ones.
Many do not even understand the difference. Natural – these are products produced by living organisms, fungi, synthetic ones are obtained completely by chemical means. Both are our weapons against microorganisms. And regardless of the way they are produced, many antibiotics cause resistance in microorganisms and thus prevent us from treating humans and animals later on. All antibiotics are toxic to some extent, it is impossible to separate them according to this principle.
Myth No. 4. Antibiotics are everywhere: in water, in bread, in the air, so there is no need to be afraid of them.
This is not entirely true. Some substances have inhibitory properties for microorganisms, but are not antibiotics. The same citric acid, the same soap. There is really no need to be afraid of antibiotics, but they need to be used wisely. And in no case should not be used for prophylactic purposes. And here we move on to the next myth.
Myth No. 5. Antibiotics should be used for prevention – this is how we prevent the disease.
5. Antibiotics should be used for prevention – this is how we prevent the disease.
Antibiotics and prevention are absolutely incompatible concepts. In no case should these drugs be used to prevent diseases.
Myth No. 6. We have been using these antibiotics for a hundred years, we are still using them, and we will live for another hundred years.
I really hope that we will live another hundred years. But you need to understand that antibiotics appeared not so long ago. And at the very beginning, every month we produced a new synthetic agent, found a new natural substance with antimicrobial properties – indeed, there were a lot of them, and we were not worried about resistance issues. “Well, this microbe has acquired resistance to penicillin – tomorrow we will come up with ceftriaxone. Acquired to ceftriaxone – came up with cefazolin. And so on – that is, updates were constantly. But today we do not have such potential. And, if we talk about absolutely new formulas, then today one, very rarely two antimicrobial agents appear in the world a year. And microorganisms are becoming more advanced, acquiring resistance to an increasing number of AMS. So we busted that myth too. We need to do something now.
And microorganisms are becoming more advanced, acquiring resistance to an increasing number of AMS. So we busted that myth too. We need to do something now.
How does the antibiotic get into milk?
There is a chain:
– Animal Blood
– Udder skin
– Skin of the milkmaid’s hands
– Utensils for milking
– Storage utensils
– Milk pipeline
– Milk cooling container
– milk carrier
– Tanker for storing milk before putting it into production (processing)
At each of the links in this chain, the antibiotic can get into the milk. And so the most basic thing we need to think about is animal health. Through their blood, the antibiotic enters the milk most often.
The second most popular source is the processing of udder skin and hand skin. Very often scratches, cracks are treated with a special solution, which is then not thoroughly washed off – or not washed off at all. As a result, a small concentration of the antimicrobial drug enters the milk – and high-precision equipment captures it.
As a result, a small concentration of the antimicrobial drug enters the milk – and high-precision equipment captures it.
The third option is deliberate stuffing. Namely, the conservation of milk in the absence of proper cooling systems. Most often this happens in personal subsidiary farms, when people do not have large industrial refrigerators. And in a regular refrigerator, a bucket does not fit. It’s good if it’s winter outside – and in summer it’s critical. It’s easier to throw half a tablet of antibiotic into milk so as not to worry that it will turn sour until morning or evening, when it will be taken for processing. Illegal? Yes. Wrong? Yes. But, if there is no punishment and it passes, people will continue to do it.
The next point is the use of synthetic antimicrobial agents in the composition of detergents and disinfectants for dishes, milk pipelines, and tanks. Not all detergents contain antibiotics, but if they are, as a result of poor washing of the container, they enter the milk. Automatic washing usually solves this problem. But if the containers are washed by hand, special attention must be paid to this item. To do this, special indicators like litmus paper are often supplied with detergents.
Automatic washing usually solves this problem. But if the containers are washed by hand, special attention must be paid to this item. To do this, special indicators like litmus paper are often supplied with detergents.
Natural contamination with fungal spores is the least likely case. But, based on my practice, it also occurs. The ingress of a small amount of microorganisms from the environment, straw, hay due to a low level of hygiene is really possible. And when the equipment shows the residual amount, when the person did not add anything, you need to understand that such an alignment is also likely, although it is really rare.
Impossible to fight antibiotics in milk until liability is spelled out
Why does the antibiotic appear in milk?
This is a belief in myths. We continue to believe in them, we are not afraid of anything, and we add an antibiotic, because it is “good and helps us.” People sometimes do not understand the real picture – that’s why they believe in myths.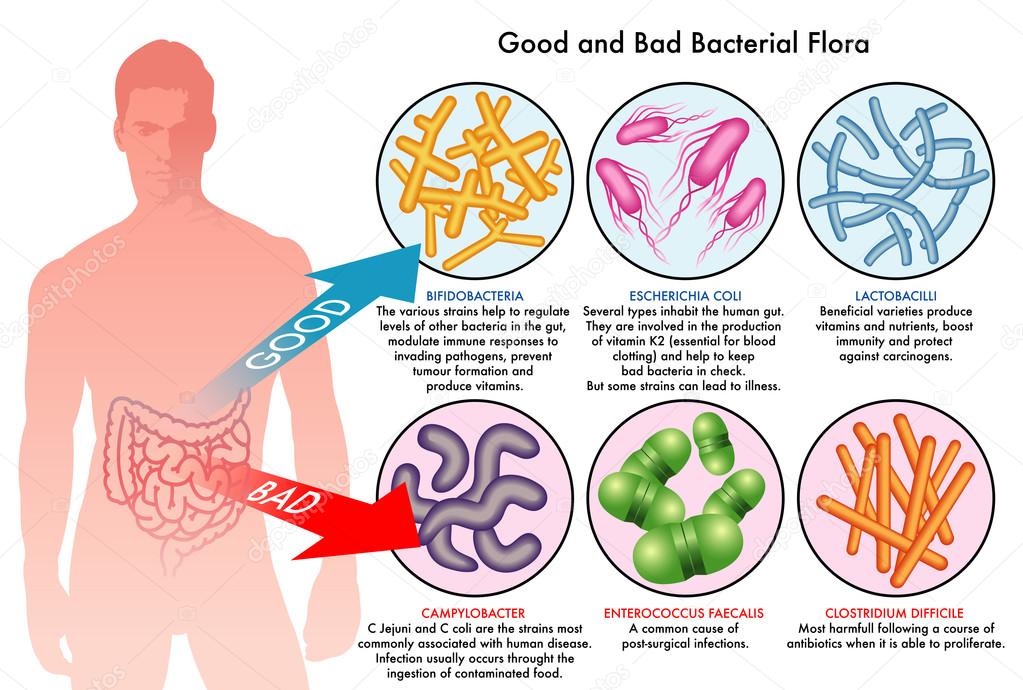 They need to be explained, told how to do it. And later it will help them to sell milk, get normal money for it and at the same time not break anything. Another factor is distorted, outdated knowledge of veterinarians. Until today, many of them think that antibiotics can be used for prevention purposes. I will say more: in textbooks on therapy, on epizootology, which are still found in our educational institutions, such an application is allowed. And this is a problem that also needs to be addressed as quickly as possible. Another reason is the unreasonable use of antibiotics: violation of the course, dosage, duration of administration. Not only do the remains of these drugs enter the milk, but also from the use of an antibiotic in the wrong doses, resistance appears in microorganisms.
They need to be explained, told how to do it. And later it will help them to sell milk, get normal money for it and at the same time not break anything. Another factor is distorted, outdated knowledge of veterinarians. Until today, many of them think that antibiotics can be used for prevention purposes. I will say more: in textbooks on therapy, on epizootology, which are still found in our educational institutions, such an application is allowed. And this is a problem that also needs to be addressed as quickly as possible. Another reason is the unreasonable use of antibiotics: violation of the course, dosage, duration of administration. Not only do the remains of these drugs enter the milk, but also from the use of an antibiotic in the wrong doses, resistance appears in microorganisms.
Plus, a very important factor is the lack of responsibility. And based on this – widespread facts of violations. If there are many violators, it is very difficult to deal with them. If responsibility is not spelled out, it is impossible to fight it.
Antibiotic resistance causes 1.3 million deaths per year
Why should antibiotics bother us? First, this is already a problem recognized by both the World Organization for Animal Health and the World Health Organization for Humans. Every year, 1.3 million people die in the world precisely for the reason that they cannot be cured with antibiotics. That is, from exposure to microorganisms that have acquired resistance. According to the WHO forecast, by 2050 the number could rise to 10 million deaths per year. This is more than in any war. The unwise use of antibiotics for animals complicates the treatment of especially vulnerable groups of the population – and these are our children, these are the elderly.
Therefore, the problem should worry us today. If you ask medical professionals, you will surely see that already today nosocomial infections are mostly resistant to antibiotics. That is, the problem is very close. And even if today this does not really bother you personally, your government, the competent authority of your country, then this worries the governments of neighboring countries. And, if you do nothing, soon you will have no one to trade with. The European Union has already introduced monitoring of antibiotic-resistant strains. The People’s Republic of China, which seeks after developed countries and is itself one, is also adapting its legislation and may introduce restrictive measures from 2026.
And, if you do nothing, soon you will have no one to trade with. The European Union has already introduced monitoring of antibiotic-resistant strains. The People’s Republic of China, which seeks after developed countries and is itself one, is also adapting its legislation and may introduce restrictive measures from 2026.
Where to start?
First, your donators need to understand the issue of antibiotic resistance. It needs to be talked about at all levels: in schools, institutes, and the media. We must ensure that the importance of the correct use of antibiotics by both medical and veterinarians is understood. This is a common problem. And when they say: “By removing antibiotics from pharmacies, you will limit our freedom,” in reality it is not so. Your health is your freedom. And limiting access to potent antibiotics is one side of the “Freedom from Antibiotic Resistance” coin. Be free from it rather than from a lack of antibiotics in pharmacies. And most importantly, never use antibiotics for prevention.
And most importantly, never use antibiotics for prevention.
What needs to be done?
1. Conduct an information campaign in the media and through local authorities among agricultural producers.
2. Free access to potent antimicrobial agents and contaminants with withdrawal periods must be unequivocally closed. It’s not easy for us either, believe me. And it won’t be easy for you. But if you prepare people for this, sooner or later it will happen, and there will be a result.
3. Introduce the One Health principle we have already talked about. Human health and animal health should no longer be separate. The Ministry of Agriculture and the Ministry of Health must work together and adhere to this principle. Just as the World Organizations for Animal Health (WOAH) and Humans (WHO) do today.
4. Legal and administrative equality of all market participants. We must remember that if we make an exception for one, no one will follow the rules. No exceptions. The requirements are the same for everyone.
No exceptions. The requirements are the same for everyone.
5. If milk with antibiotics appears on the market, we must know what to do with it. The procedure for the disposal of such milk according to the “counterfeit banknote” principle must not only be developed and implemented – it must also be carried out. If you recycle one, two, three, five, 20 batches – believe me, the 21st may not appear. People will understand: no one needs this milk, it cannot be used. With the existing shortage of raw materials, even the most “dirty” milk finds its processor. And then the consumer.
6. Therefore, one more point is mass informing the consumer through rating channels and print media about the existing problem and creating a healthy hype around the products of reputation-responsible manufacturers. Let them get super profits rather than tomorrow we get a weak generation. Conscientious producers should be advertised by the state, if they were able to achieve pure milk – let people vote for them with a ruble.
7. If you want to be better than others, create your own industry standard that is higher than the state standard. Take milk according to this standard. Prove that your chain is free of antimicrobials. And the consumer will appreciate it, even if your products are a little more expensive. And if you also get into the price corridor, you are just at the peak. But, once again, the state should help you – without this, unfortunately, nothing can be done.
8. Improving training programs for veterinarians is an unequivocal truth that needs to be addressed today. Borrow the educational program from those countries where it already exists, translate it into your language, give students the information they deserve, which will help them work not only in your country, but in any other.
9. State monitoring of the circulation of not only residual amounts of AMS, but also antibiotic-resistant strains.
10. Dairy module: selection program based on the principle of surprise and state monitoring of raw milk in terms of basic indicators: microbial count, somatics, 4 groups of antibiotics. This is a very important issue that you must implement if you want to trade with other countries.
This is a very important issue that you must implement if you want to trade with other countries.
Thank you for your attention. I wish you to be healthy, happy and have a lot of healthy, high-quality milk. And most importantly, a healthy generation. Our future is our children.
Peptide antibiotics isolated from moon milk
Belgian scientists studied the genome of streptomycetes from plaque on cave walls, which is called moon milk, and discovered the genes of an enzyme system that synthesizes peptide antibiotics from a previously unknown chemical group. The scientists reconstructed the chemical structure of the peptides, dubbed lunemycins, and tested them against bacteria that cause infections in humans. The compounds have shown to be effective agents against Gram-positive bacteria. The study was published in the journal International Journal of Molecular Sciences .
Bacterial resistance to antibiotics is already one of the top three causes of death in the world (we talked about the problem of antibiotic resistance in the article “The End of a Beautiful Era”). Ultimately, resistance will develop to any antibiotic – it’s only a matter of time. Therefore, scientists are constantly looking for new antimicrobial compounds – sometimes in silico , and sometimes in ecosystems where many bacteria live, forced to compete with each other for scarce resources.
Ultimately, resistance will develop to any antibiotic – it’s only a matter of time. Therefore, scientists are constantly looking for new antimicrobial compounds – sometimes in silico , and sometimes in ecosystems where many bacteria live, forced to compete with each other for scarce resources.
Cave walls are an example of such a community. In order to survive in a cave, one must either be extremely resistant to natural changes or develop antibiotics. Streptomycetes, gram-positive bacteria from the actinobacteria group, were especially successful in the development of antimicrobial agents. Most streptomycete strains synthesize up to several tens of antimicrobial compounds simultaneously, and up to several tens of percent of the genome is responsible for their synthesis.
One of the microbial bacterial cave communities, moonmilk, is largely composed of streptomycetes. Back in the Middle Ages, it was used in folk medicine for the treatment of infectious diseases of the skin and eyes in humans and domestic animals.
Belgian microbiologists and chemists led by Sebastien Rigali from the University of Liege investigated the metabolome and genome of one of the microorganisms that make up moon milk in the Belgian cave Grotte des collemboles. They collected three samples of raids from the walls of the cave and isolated 18 strains from them belonging to the species Streptomyces lunaelactis (the species was discovered several years earlier by the group of Professor Rigali).
After sequencing their genome, the scientists found that different strains have a common gene cluster encoded in the plasmid. This region of the genome (called BGC28a) consisted of approximately 70 kb and encoded a multisubunit enzyme complex from the family of nonribosomal peptide synthetases. The cluster included genes whose products, judging by the analysis of their sequence, made it possible for streptomycetes to synthesize the D-isomer of phenylalanine and the piperazic amino acid not encoded by the genome.
Having received evidence that bacteria have a sufficient genetic apparatus for the synthesis of antimicrobial non-ribosomal hexapeptides, scientists began to search for the compounds themselves. They grew cultures of several strains of streptomycetes and, using high-resolution mass spectrometry, found 15 compounds with a molecular weight in the range of 700-800 daltons. They named the new group of antimicrobial peptides lunemycins. Mass spectrometry showed that among them the substance with the gross formula C 35 H 52 N 10 O 7 , named lunemycin A.
Scientists selected three lunemycins that dominated the S. lunaelactis metabolome 90 149 (lunemycins A, B1, D) and studied their antimicrobial activity against gram-positive and gram-negative bacteria. The results showed that the antibiotic is effective against gram-positive bacteria, but ineffective against gram-negative ones. The minimum inhibitory concentration for various molecules and pathogens lies in the range of 0.

 Dairy products include milk as well as butter, yogurt, and cheese. After taking an antibiotic you may need to wait for up to three hours before eating or drinking any dairy products. Grapefruit juice and dietary supplements containing minerals like calcium may also work dampen the effect of antibiotics.
Dairy products include milk as well as butter, yogurt, and cheese. After taking an antibiotic you may need to wait for up to three hours before eating or drinking any dairy products. Grapefruit juice and dietary supplements containing minerals like calcium may also work dampen the effect of antibiotics. Antibiotics might interact with some blood thinners and antacids, for example. Some antibiotics can make birth control pills less effective.
Antibiotics might interact with some blood thinners and antacids, for example. Some antibiotics can make birth control pills less effective. Taschenlehrbuch Medizinische Mikrobiologie. Stuttgart: Thieme; 2014.
Taschenlehrbuch Medizinische Mikrobiologie. Stuttgart: Thieme; 2014.